Abstract
Enterococcus cecorum is a well-known component of the normal poultry intestinal microbiota and an important bacterial pathogen. Infections caused by E. cecorum have negative effects on the poultry production worldwide. In this study we used the SPF-chicken embryo lethality assay (ELA) to assess the pathogenic potential of E. cecorum. A total of 23 isolates were used: 19 clinical isolates from field outbreaks in different poultry groups (CB – broiler chickens, BB – broiler breeders, CL – layers, T– turkeys, W – waterfowl) and 4 commensal isolates. The cumulative mortality caused by all clinical isolates was higher (53.4%) than that of the commensals (38.9%). The highest mortality was induced by CB isolates (68.9%), followed by CL (60.4%), all chicken isolates (59.2%; CB, BB, CL), BB (45.8%), T (41.7%), non-chicken isolates (40.7%; T, W), and W isolates (39.8%). Most of the embryos that died, did die on the 1st day post-infection (dpi), except those infected with CB, CL (on 2 dpi). The median lethal dose (LD50) of E. cecorum ranged from 6.07 × 102 cfu/ml (CB isolates) and 1.42 × 104 cfu/ml (all clinical isolates) to 4.8 × 105 cfu/ml (commensal isolates). This study provides the first evidence of a wide tissue distribution and multiplication of E. cecorum in embryos. Dead embryos showed scattered petechiae, hemorrhages, aggregates of bacteria in blood vessels, multiple organ necrosis, and encephalomalacia. Our data indicate that surviving embryos were able to elicit innate immune response to infection. On the other hand, reisolation of viable bacteria from surviving embryos may suggest that E. cecorum could evade or resist immune mechanisms in order to persist in organs. Furthermore, body mass of surviving embryos was affected by the strain type, not the dose (bacterial concentration) used, and was lower for the infection with clinical strains. The results indicated the highest pathogenicity of clinical E. cecorum isolates from CB and CL flocks.
Similar content being viewed by others
Introduction
Enterococcus cecorum is a facultatively anaerobic, Gram-positive, catalase-negative and pyroglutamic acid arylamidase (PYRase)-negative bacterium1,2. During the last two decades, E. cecorum gained recognition in the field as an emerging pathogen responsible for significant losses in the poultry industry. The morbidity and mortality can vary across countries and flocks. Overall 25–35% of the broiler flock can be affected, with 5–15% mortality and nearly 10% culling rate. To date, the economic costs of E. cecorum infections have not been calculated and specified3,4,5,6,7,8,9,10. The particular predisposition to infection of meat-type chickens and the sensitivity of other poultry species to infection have been confirmed2,11,12,13. Outbreaks of E. cecorum infection have been reported worldwide in broiler chickens (CB), broiler breeders (BB), laying hens (CL), waterfowl (W), turkeys (T), and racing pigeons2,3,4,7,10,14,15,16. Studies have shown that the disease occurs in different age groups depending on the type of poultry. Some differences have also been demonstrated in terms of the clinical manifestation of infection and frequency of E. cecorum isolation from the affected tissue samples depending on the poultry group, which may suggest differences in pathogenesis12. Moreover, some differences have been noted with regard to the type of lesions depending on the poultry group, even after experimental infection11,12. E. cecorum has been implicated in various pathological conditions including vertebral osteomyelitis, femoral head necrosis, fibrinous pericarditis, fibrinous hepatitis, fibrinous pneumonia, fibrinous ovaritis, hydropericardium, and septicemia3,4,6,8,12,14. Joint, heart and liver lesions have been observed in all poultry groups, but spinal lesions have been reported only in broiler chickens and broiler breeder chickens12. The worldwide increase in the disease outbreaks may suggest the conversion of specific strains from commensal to pathogenic and the emergence of strains with increased virulence potential5,6,17. The roles of specific concurrent infections, unspecified (host-related and environmental) predisposing factors, and changes in genetics have also been discussed3,4,17,18. Our previous research demonstrated genetic heterogeneity among clinical E. cecorum strains derived from poultry12. Strains responsible for the recent emergence of associated enteroccocal diseases showed high levels of antimicrobial resistance and virulence determinants17,19. However, the prevalence of virulence determinants among the European pathogenic strains was relatively low12,13. The differences in genomic features, phenotypic properties and colonization abilities between commensal and pathogenic isolates may indicate their divergent evolution9,13,19,20.
The purpose of this study was to evaluate the virulence of clinical E. cecorum isolates from different poultry groups by using experimental infection of chicken embryos. Even though many reports on the use of chicken embryo lethality assay (ELA) in assessing the pathogenic potential of bacterial pathogens have been published21,22,23,24, only three referred to E. cecorum13,25,26, while most focused on E. faecalis or E. faecium22,27,28,29,30. Therefore, we aimed to determine the median lethal dose (LD50) of E. cecorum from different poultry groups and to identify pathomorphological lesions in embryos, preferential tissue distribution and bacterial load.
Methods
Bacterial strains
A total of 23 E. cecorum isolates were used in this study: 19 clinical (pathogenic) strains previously isolated from independent field infection cases in poultry between 2011 and 2017 in Poland (Supplementary Table 1), 4 commensal strains from the ceca of healthy chickens, including 1 type strain (ATCC 43,198) from chicken ceca. Clinical field isolates represented different poultry species, production groups, flocks (5 chicken broilers – CB, 4 broiler breeders – BB, 4 layers – CL, 3 turkeys – T, 3 waterfowl – W), years, geographic locations, and clones (based on the PFGE pulsotypes). Isolates were retrieved from tissues demonstrating pathological lesions and characterized in our previous study12.
Inoculum preparation
All bacterial cultures were incubated overnight microaerobically at 37 °C on Columbia agar plates with sheep blood (CA, Graso, Poland). The stock solutions were prepared by dissolving colonies of each E. cecorum isolate (n = 23) in saline to match the turbidity of 1.0 McFarland standard (DEN-1, Biosan, Riga, Latvia), which corresponds to approximately 3.4 × 108 colony-forming units/ml (cfu/ml). The bacterial concentration was confirmed for each isolate using the standard enumeration method, by spreading 100 μl of dilutions on triplicate Enterococcosel agar plates (Graso, Poland). The stock solution and eight dilutions (up to 10–8) of each isolate were used for inoculation.
Experimental design
The ELA was performed on fertile specific pathogen–free (SPF) chicken eggs (VALO BioMedia GmbH, Germany) according to the procedure adopted from the literature25,31. Before inoculation, the eggs were candled to determine embryo viability, then the air cell, large blood vessels, and embryo location were marked. The eggshell injection site (over the air cell) was disinfected with iodine and penetrated with a sterile needle. Groups of 11-day-old embryonated SPF eggs were inoculated into the chorioallantoic sac (CAS) with 0.1 ml of each bacterial solution (4 eggs were used per each dose). In addition to the infected embryos, there was a control group which consisted of 4 embryos inoculated with sterile saline and another one non-inoculated. Every inoculation was done with a sterile 1 ml syringe (Medical – Łomża, Poland) and a 23G needle (0.6 × 25 mm). After inoculation, the opening was sealed with a small piece of tape. All eggs were then incubated in the same incubator (Heka Incubator, Przewoz, Poland) at 37 °C, 55% humidity, without turning, for 7 days with daily candling to determine the embryo mortality rate (EMR). Embryos that survived until the 7th day post-infection (dpi), i.e. until the 18th day of incubation (di), were chilled at 4 °C for one hour.
Median lethal dose (LD50)
The LD50 was calculated using the Reed and Muench method based on the cumulative number of dead embryos and surviving embryos at each dilution32,33. The cumulative mortality caused by all E. cecorum strains of each poultry group were used for calculations.
Pathomorphological lesions
Dead and survived embryos were necropsied for gross lesions. Fresh tissue samples were collected from embryos on the day of necropsy and fixed in 10% buffered formalin. The tissue samples were submitted to the Division of Animal Pathology, Department of Pathology and Veterinary Diagnostics, Institute of Veterinary Medicine at the Warsaw University of Life Sciences—SGGW, Poland, for histopathological examination. The samples were routinely processed: dehydrated in increasing gradients of ethyl alcohol, embedded in paraffin and cut into 4 µm-thick sections using a microtome. Subsequently, paraffin sections were stained with hematoxylin and eosin (HE). The samples were analysed under the BX41 light microscope (Olympus, Japan).
Reisolation of E. cecorum
The specific organ samples were tested separately for each E. cecorum isolate. Yolk sac, heart, and gizzard (muscular stomach) samples from dead (on 13 di, 2 dpi) and surviving (on 18 di, 7 dpi) embryos that were inoculated with the highest bacterial concentration (approx. 3.4 × 107 cfu/egg) were used for reisolation of E. cecorum. Tissue samples were cut using a sterile scalpel blade and homogenized with sterile saline on a vortex mixer until complete disruption and obtaining homogeneous tissue suspensions. The plate count method (on Enterococcosel agar, Graso, Poland) was used to determine the total number of bacteria found in the organs mentioned. The total number of bacteria was expressed as cfu/g of organ (yolk sac, heart, gizzard) and cfu/heart (total heart weight). Identification of reisolated E. cecorum was confirmed by colony morphology on CA plates, catalase test or PCR with species-specific primers34.
Body mass of embryos
Embryos that survived infection until the last day of the experiment (7 dpi, 18 di) were weighed using an Ohaus® PA214CM/1 scale (max: 210 g, min: 0.01 g, e = 0.001 g, d = 0.0001 g, Ohaus®, USA).
Statistical analysis
Numerical variables were presented as an arithmetic mean and standard deviation (SD), or a median and interquartile range (IQR), depending on the shape of their distribution. Range was given in all cases. Numerical variables were compared between unpaired groups with the Mann–Whitney U test, and between paired groups using the Friedman or Wilcoxon signed rank test, with the Bonferroni correction in the case of multiple comparisons. Categorical variables were given as counts and percentages, which were then compared between groups using the Pearson χ2 test. The ninety five per cent confidence intervals (95% CI) for proportions were calculated according to the Wilson score method. When more than two groups were compared, the χ2 test was considered an omnibus test. Therefore, when it yielded a significant result, a post-hoc analysis was performed according to the procedure described by Markowski and Markowski35. Briefly, the group with the largest average contribution to the χ2total was identified and removed from the contingency table, and the χ2 test was performed again. The procedure was repeated until the χ2 test yielded an non-significant result.
Embryo survival probability was analysed using the Kaplan–Meyer plots and compared between groups with the log-rank test. The influence of the dilution (dose) and the type or groups of strains on mortality was investigated using the multivariable logistic regression model, and their role was presented as odds ratio (OR). The influence of the dilution and the type or groups of strains on the body mass of embryos (YBM) was investigated using the general linear model (GLM) with dilution and clinical type of strains (Xclinical) (model 1) or dilution and groups of strains (XCB, XBB, XCL, XT, XW) (model 2) fitted in the GLM as fixed factors, and commensal strains serving as a reference category. GLMs were expressed with the equation:
B0 was the intercept, and B with a relevant subscript stood for the coefficient of regression of a given explanatory variable.
All statistical tests were two-sided. The significance level (α) was set at 0.05. Statistical analysis was performed in TIBCO Statistica 13.3 (TIBCO Software Inc., Palo Alto, CA, USA). GLMs were developed in IBM SPSS Statistics 26 (IBM Corporation, Armonk, NY).
Ethics approval
The Approval of Animal Ethics Commission was not required for the presented work according to the Polish law (the Act on the Protection of Animals Used for Scientific or Educational Purposes of 15 January 2015, published in the Journal of Laws of 2015 as item 266) and the European Union regulations (Directive 2010/63/EU of the European Parliament and of the Council of 22 September 2010 on the protection of animals used for scientific purposes). The experiment on SPF chicken embryos was completed 3 days prior to hatching, on developmental day 18 at the latest. All methods were carried out in accordance with the relevant guidelines and regulations. The study was carried out in compliance with the ARRIVE guidelines.
Results
Embryo mortality rate
EMRs for each dose of clinical and commensal E. cecorum strains are shown in Table 1. Analysis of the effect of strain type and dose on EMR showed that mortality decreased along with decreasing infective dose, both in clinical and commensal E. cecorum strains (p < 0.001). Infection with clinical strains doubled mortality at all doses compared with infection with commensal strains (p = 0.001, OR 1.90, 95% CI: 1.29, 2.79). Dose–response analysis showed that infection with strains belonging to groups CB and CL significantly increased mortality, roughly 2–4 fold, compared with commensal strains (CB p < 0.001, OR 3.47, 95% CI: 2.37, 5.08; and CL p < 0.001, OR 2.31, 95% CI: 1.55, 3.44). Infection with strains from the remaining groups was not significantly linked to mortality compared with commensal strains (BB p = 0.209, OR 1.30, 95% CI: 0.86, 1.96; T p = 0.658, OR 1.12, 95% CI: 0.69, 1.80; and W p = 0.876, OR 1.04,95% CI: 0.61, 1.78).
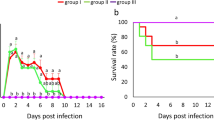
The cumulative EMR determined for all E. cecorum strains at all doses is presented in Fig. 1A–C, Table 2, Supplementary Table 2, and Supplementary Table 3. The cumulative EMR caused by all clinical strains was estimated at 53.4% with 365 deaths of 684 embryos (95% CI: 49.6, 57.1) and was significantly higher (p = 0.002) than mortality caused by all commensal strains: 38.9% (95% CI: 31.3, 47.0; 56/144) (Fig. 1B). The cumulative EMR was significantly higher in embryos inoculated with E. cecorum strains from CB flocks: 68.9% (95% CI: 61.8, 75.2; 124/180; p < 0.001) and CL flocks: 60.4% (95% CI: 52.3, 68.0; 87/144; p = 0.002) than in those inoculated with E. cecorum from other poultry types – BB: 45.8% (95% CI: 37.9, 54.0; 66/144), T: 41.7% (95% CI: 32.8, 51.1; 45/108), and W: 39.8% (95% CI: 31.1, 49.2; 43/108) (Fig. 1A). The cumulative EMR was significantly higher (p < 0.001) after infection with clinical chicken strains (CB, BB, CL): 59.2% (95% CI: 54.7, 63.5; 277/468) than with other poultry strains (T, W): 40.7% (95% CI: 34.4, 47.4; 88/216) and commensal strains: 38.9% (Fig. 1C). The comparison of cumulative EMR between E. cecorum strains (χ2 test) within the same poultry group showed significant differences for BB, T, and W (p < 0.001), and no differences for CB (p = 0.34), CL (p = 0.057), and commensal (p = 0.972) groups.
Cumulative embryo mortality rate (%) following infection with all doses of (A) clinical CB (n = 5), BB (n = 4), CL (n = 4), T (n = 3), W (n = 3) and commensal (n = 4) Enterococcus cecorum strains (B) all clinical (n = 19) and commensal (n = 4) E. cecorum strains (C) all clinical chicken isolates (n = 13; CB, BB, CL) and commensal E. cecorum. **p < 0.001, *p < 0.05.
Table 2 shows the summary of embryo mortality by day and cumulative mortality. Daily EMR was significantly lower in commensal strains than in other groups on day 1 (p = 0.030) and it was higher in CB and CL strains than in other groups on day 2 (p < 0.001). EMR was higher in all clinical strains than in commensal strains on day 1 (p = 0.003) and 2 (p < 0.001) (Supplementary Table 2), but it was lower on day 5 (p = 0.009). EMR was lower (p = 0.010) in commensal strains than in chicken and other poultry strains on day 1. EMR was higher in chicken strains than in other poultry strains and commensal strains on day 2 (p < 0.001) (Supplementary Table 3). EMR differed (p < 0.001) between days (from 1 to 7 dpi) within each group of E. cecorum strains (in columns: Table 2, Supplementary Table 2, Supplementary Table 3). No mortality was observed in the control group.
Embryo survival
Survival rate on 7 dpi was significantly lower in embryos infected with clinical strains (46.6%) than in those infected with commensal (61.1%) strains of E. cecorum (log-rank test p = 0.001) (Fig. 2A). Among clinical E. cecorum strains, survival rate on 7 dpi was the lowest in CB (31.1%), followed by CL (39.6%), BB (54.2%), T (58.3%), and W (60.2%) strains. A total of 46.6% embryos survived after infection with clinical strains, 41.4% after infection with broiler (CB, BB) strains, and 40.8% after infection with chicken (CB, BB, CL) strains. Survival rate on 7 dpi was significantly lower in embryos infected with chicken strains (CB, BB, CL) (40.8%) than in embryos infected with other poultry (T, W) strains (59.3%) and commensal strains (61.1%) (Generalized log-rank test p < 0.001) (Fig. 2B).
LD50
The LD50 for E. cecorum ranged from 102 to 105 cfu/ml (Table 3) and was the lowest in CB isolates. Compared with commensal isolates, LD50 was approx. 800-fold lower in CB isolates, 130-fold lower in chicken isolates (CB, BB, CL), and 34-fold lower in all clinical E. cecorum isolates.
Pathomorphological Lesions
The most prominent macroscopic lesions occurred in embryos infected with the highest doses (approx. 3.4 × 107 cfu/egg, 3.4 × 106 cfu/egg) of clinical E. cecorum that died on 13 di (2 dpi). No lesions were observed in the embryos of the control group. Embryos that died until 15 di (4 dpi) inclusive after infection with chicken isolates (CB, BB, CL) showed severe macroscopic lesions: generalized body congestion, multifocal hemorrhages in the pectoral and thigh muscles, and petechial hemorrhages beneath the epicardium, in the gizzard wall, and in the proventriculus mucosa (Fig. 3). In addition, an abnormal yolk sac content (cloudy, dense, greenish) was noted. The liver was enlarged, friable, congested or greenish in color. The kidneys were congested, the spleen was enlarged, congested or pale, the bursa of Fabricius was enlarged. Embryos infected with commensal isolates showed no or less pronounced lesions. In addition, the surviving embryos (on 7 dpi, 18 di) showed no lesions, but single embryos (receiving the high dose) had abnormal spleen, liver, or yolk sac.
Pathomorphological lesions of SPF chicken embryos (1–4 dpi) inoculated with Enterococcus cecorum strains. (A) Comparison between congested embryos (2 dpi) infected with clinical E. cecorum and embryo infected with commensal (*) E. cecorum (approx. 3.4 × 106 cfu/egg) (B) Hemorrhages of skeletal muscles in embryos infected with clinical E. cecorum. (C) Hemorrhages on the head and petechiae on the legs (4 dpi) (D) Petechiae on the skull of embryos infected with clinical E. cecorum. (E) Petechiae on the epicardium of embryos infected with clinical and commensal (*) E. cecorum isolates. (F) Petechiae on the gizzard (3 dpi) and (G) on the mucosa of proventriculus (7 dpi) of embryo infected with clinical E. cecorum. (H) Hemorrhages on thigh muscles in embryos (2 dpi) infected with clinical (CB, CL, respectively) and commensal (*) E. cecorum isolates (approx. 3.4 × 107 cfu/egg).
Histopathological examination of the tissue samples of dead embryos showed aggregates of bacteria in blood vessels in the liver, kidney, heart, gizzard, and brain, multiple areas of necrosis in the liver and heart, and necrosis of renal tubular epithelial cells and gizzard smooth muscle cells. In addition, dead embryos showed encephalomalacia, hemorrhages in the liver, kidney, and heart, as well as marked congestion of the proventriculus, gizzard, and brain (Fig. 4). Tissue samples from the surviving embryos revealed inflammatory response (hepatitis, glomerulonephritis, pericarditis, and proventiculitis), glial and endothelial cell reaction in the brain, and mainly mild to moderate congestion. Bacteria were not found in blood vessels (Fig. 5A–F). Embryos infected with commensal strains showed a mild degree of histopathological lesions (mainly congestion, hepatocyte degeneration, and mild infiltration consisting of heterophils and mononuclear cells) (Fig. 5G–H). Figure 5I shows a representative image of the control group.
Histopathological lesions in chicken embryos that died (on 13 di, 2 dpi) following infection with clinical Enterococcus cecorum strains. HE stain. (A) Liver: severe congestion, degenerated and necrotic hepatocytes (circles), loosely arranged hepatic trabeculae, bacterial colonies in blood vessels indicated by arrowhead. Bar: 20 µm. (B) Kidneys: severe congestion, hemorrhages (*), capillary blood vessels of glomeruli with bacteria (arrowheads), degenerated and necrotic epithelial cells of renal tubules. Bar: 20 µm. (C) Heart: multifocal necrotic areas (circles), massive hemorrhages (*) and severe congestion. Bar: 200 µm; Insert: blood vessels with bacterial aggregates, necrotic cardiomyocytes. Bar: 20 µm. (D) Proventriculus. severe congestion (*) and edema of the stroma. Bar: 50 µm. (E) Gizzard: diffuse necrotic muscle fibers. Arrowhead indicated blood vessels with bacteria in the muscle layer. Bar: 20 µm. (F) Brain: bacterial aggregates (arrowhead) in the blood capillary, pyknosis of glial nuclei and cerebral malacia. Bar: 20 µm.
Histopathological lesions in chicken embryos that survived (7 dpi, 18 di) infection with clinical (A–F) and commensal (G–H) Enterococcus cecorum isolates and in non-infected embryos of the control group (I). (A) Liver: multifocal areas infiltration of heterophils and mononuclear cells (arrows), necrotic areas (circle), moderate congestion (*). Bar: 200 µm. Insert: moderate degree of inflammatory infiltrate, mild vacuolization of hepatocytes and increased number of bile pigment-laden macrophages. Bar: 20 µm. (B) Kidneys: diffuse markedly enlarged glomeruli, vacuolization and necrosis of epithelial cells. Arrows indicated inflammatory cells (heterophils and lymphocytes). Bar: 20 µm. (C) Heart: diffuse infiltration of pericardium and epicardium with heterophils and mononuclear cells. Bar: 200 µm. Insert: Bar: 50 µm. (D) Proventriculus: mild infiltration of inflammatory cells (heterophils and mononuclear cells) in the tunica intima and between deep glands, edema of the stroma. Bar: 200 µm. (E) Gizzard: there was no evidence of inflammation. Bar: 50 µm. (F) Brain: severe gliosis, capillary hyperplasia and endothelial hypertrophy, moderate neuropil vacuolization. Bar: 20 µm. (G) Liver: mild vacuolization of hepatocytes and infiltration of heterophils and mononuclear cells (arrow) around blood vessels. Bar: 50 µm. (H) Gizzard. No presence of inflammation. Bar: 50 µm. (I) Heart: no presence of inflammation. Bar: 50 µm.
Reisolation of E. cecorum after Infection
Pure cultures of E. cecorum were reisolated from inoculated embryos, but none of the control group. E. cecorum was reisolated from all organs (yolk sac, heart, gizzard) of dead embryos (on 13 di, 2 dpi) and from all organs except gizzard (16.7%) of surviving embryos (on 18 di, 7 dpi).
The number of reisolated E. cecorum from the whole heart (cfu/heart) did not significantly differ between clinical and commensal strains in dead embryos and surviving embryos (Table 4) (it was higher in a non-significant manner in commensals). Bacterial loads in the whole heart of embryos that died (on 2 dpi) after inoculation with approx. 3.4 × 107 cfu/egg of E. cecorum reached approx. 3.73 × 107 cfu for clinical strains and approx. 10.5 × 107 cfu for commensal strains. The number of reisolated E. cecorum from the whole heart of surviving embryos (on 7 dpi) was markedly lower and reached approx. 6.57 × 102 cfu for clinical strains and 18.3 × 102 cfu for commensal strains.
The comparison of the results of bacterial load (cfu/g) between the organs of dead and surviving embryos is shown in Table 5. Reisolation (cfu/g) was significantly higher in all organs of dead embryos compared with that in surviving embryos for clinical (p < 0.001) and commensal (p = 0.029) strains. The difference in bacterial load (cfu/g) between the organs of dead embryos was significant only for commensal E. cecorum strains (p = 0.039) (Supplementary Table 4). Reisolation of E. cecorum from surviving embryos was significantly higher in the yolk sac than in the heart (p < 0.001) or gizzard (p = 0.015) only for clinical strains (Supplementary Table 5). Bacterial loads in organs did not differ significantly between clinical and commensal strains in embryos that died (on 13 days, 2 dpi) (Supplementary Table 4) and in those that survived infection (on 18 di, 7 dpi) (Supplementary Table 5).
Body mass
Results of body mass were presented without the yolk sac contents (yolk-free embryo weight). The inoculating dose did not influence the body mass of embryos. Body mass was significantly lower (p = 0.006) in embryos infected with clinical strains (21.61 g; 95% CI: 21.39 g, 21.82 g) compared with those infected with commensal strains (22.29 g; 95% CI: 21.85 g, 22.72 g) (Supplementary Table 6). Group of strains (CB, BB, CL, T, W, commensal) had a significant impact (p = 0.027) on the body mass of surviving embryos. Body mass was significantly lower in embryos infected with clinical strains from CB (21.44 g; 95% CI: 20.93 g, 21.95 g; p = 0.013), T (21.54 g; 95% CI: 21.08 g, 22.01 g; p = 0.022) , and W (21.33 g; 95% CI: 20.87 g, 21.78 g; p = 0.003) compared with those infected with commensal strains (22.29 g; 95% CI: 21.85 g, 22.72 g) (Fig. 6).
Discussion
The current study investigated the effect of infection of SPF chicken embryos with clinical E. cecorum isolates from five different poultry groups (CB, BB, CL, T, W), as well as with commensal E. cecorum (C), on embryo survival, pathomorphological lesions, body mass of surviving embryos, and bacterial growth kinetics in embryos.
ELA has demonstrated the ability to discriminate between virulent and avirulent avian Escherichia coli and Rimerella anatipestifer isolates36,37. Previous reports have suggested that ELA may also be a useful method in distinguishing pathogenic and commensal E. cecorum strains25. Jung et al. observed higher mortality caused by poultry pathogenic E. cecorum strains than by commensal strains (approx. 40% vs. 20%)13. Furthermore, analysis of E. cecorum isolates from various species (chicken, ducks, goose, turkeys, pigeons, budgerigar, swan, cattle, swine, human) showed higher mean total embryo lethality caused by pathogenic strains compared with commensal strains (39.7% vs. 18.9%)13. Considering that the cumulative EMR caused by poultry clinical E. cecorum strains in our study was significantly higher than that caused by commensal strains (53.4% vs. 38.9%), and EMR caused by chicken strains (CB, BB, CL) was significantly higher than that caused by commensal strains (59.2% vs. 38.9%), our findings support the previous reports. But in fact, clinical E. cecorum strains from CB and CL were primarily responsible for this high mortality. According to our best knowledge there is no data concerning the pathogenicity of clinical E. cecorum from BB and CL. The only one report concerned commensal strains from CL, and EMR was much lower (approx. 17%) than that provided for clinical CL in our study (60.4%)13.
Considering that E. cecorum infection is one of the most important bacterial diseases for broiler and broiler breeder flocks (meat chickens)3,4,6,8,12,14, we were surprised by the relatively low EMR caused by BB strains (6.9% difference between BB and C is actually a 17% increase for BB over the 38.9% obtained for C). Infection with CB and CL strains caused a significantly higher (approximately twofold) overall EMR compared with commensal strains. Infection with strains from the remaining groups of poultry (BB, T, W) did not produce any significant cumulative EMR compared with commensal strains. Other authors reported no statistically significant ELA results of comparisons between a pathogenic E. cecorum strain from broiler and a control (phosphate-buffered saline); however, the variable results for some isolates may have arisen from using different sets of eggs26. Although T and W strains caused a lower EMR than CB strains, it was higher than that reported for turkey strain and similar to waterfowl strains13. This may indicate a lower pathogenicity of T and W strains, but it may also imply some host species specificity of E. cecorum strains. The literature data showed differences in susceptibility between broiler and layer embryos and a low mortality of chicken embryos caused by human E. cecorum13,26. This finding may support the necessity for use of species-specific embryos in ELA.
As expected, mortality decreased significantly along with decreasing infectious dose. The effect of poultry group and dose on mortality was demonstrated by the significantly higher EMR for CB and CL isolates compared with commensal isolates. However, in some poultry groups, injection of a lower dose was not always associated with the parallel reduction in mortality. Similarly, Ekesi et al.26 showed that a lower dose (105 cfu/ml) of pathogenic E. cecorum produced slightly more lethality than a higher dose (106 cfu/ml), but neither was statistically different from the PBS control. Other authors reported that some E. faecalis strains with the lowest dose (cfu/ml) were able to produce high embryo mortality, whereas other E. faecalis strains with a higher dose produced low mortality29.
In our opinion, there may be a large variation in EMR between isolates, and ELA may show lower discriminative power. The low pathogenicity of distinct bacterial species including Staphylococcus spp., E. cecorum, E. coli isolated from lame broilers with bacterial chondronecrosis with osteomyelitis (BCO) suggested that ELA might not be an effective measure for assessing bacterial virulence with respect to BCO26. Our results revealed the uniformity of EMR among E. cecorum isolates within CB, CL, and the commensal group. On the other hand, the strain differences within the BB, T, W groups could have influenced the ELA results. The within-group differences may explain the lower EMR in these groups.
Our study demonstrated significant differences in the survival of embryos infected with clinical and commensal E. cecorum strains (46.6% vs. 61.1%). Moreover, survival decreased significantly in embryos infected with clinical chicken strains (CB, BB, CL) compared with other poultry (T, W) strains and commensal strains. Similar observations were described previously for broiler isolates25. In contrast to the reduced survival of SPF embryos (9%) and non-SPF broiler embryos (23%) infected with pathogenic E. cecorum isolates from broilers in the southeastern United States25, the survival rates of embryos infected with pathogenic broiler strains from Poland (this study) and Germany13 were higher. This may suggest a lower pathogenicity of European isolates. In contrast, the embryo survival was similar for the commensal E. cecorum strains from our study and those from southeastern United States25.
Early embryo mortality due to E. cecorum inoculation has been observed in previous studies13,25,26. The phenomenon may allow drawing meaningful conclusions with respect to the relative pathogenicity of strains38. In our study, most of the infected embryos died on 1 dpi, and then EMR gradually decreased. The exception are the CB and CL strains for which EMR was even higher on 2 dpi, and then declined. In the literature, the highest embryo mortality on 2 dpi has been recorded for avian pathogenic E. coli (APEC) isolates39,40 and on 3 and 4 dpi for avian E. faecalis29. Massive and rapid (within 2 days) mortality has also been noted for avian pathogenic E. faecalis in yolk sac-inoculated embryos41. It is noteworthy that in our study, E. cecorum strains from T failed to cause mortality after 4 dpi, while other clinical as well as commensal strains caused single deaths on 7 dpi. In contrast to our results, commensal E. cecorum strains in another study failed to cause mortality after 2 dpi25.
There is limited data on the LD50 value of E. cecorum. According to the only available results, the 102 dose was the lowest one that reliably achieved mortality greater than 50%25. Compared with the previous report, our findings provide LD50 values for clinical E. cecorum of different poultry and commensal strains. Lower LD50 values were obtained for clinical chicken strains and all poultry strains than for commensal strains. As lower LD50 is indicative of increased toxicity, we conclude that E. cecorum from CB (102 cfu/ml) and CL (103 cfu/ml) showed the highest virulence. The LD50 of pathogenic CB E. cecorum isolates in our study was approximately 90-fold lower than the LD50 (6.6 cfu/ml) of a single avian pathogenic E. faecalis strain22, which may suggest lower pathogenicity of E. cecorum than that of E. faecalis; however, further research is needed to confirm and expand on this finding. The literature review indicated that the LD50 of E. cecorum is lower than that of avian Mycoplasma gallisepticum and some Mycoplasma synoviae strains38,42. It has been reported for Salmonella Gallinarum that isolates with a Log10LD50 of ≤ 4.0 should be considered to be virulent43. In our study, CB, CL, and all chicken strains (with log10LD50 2.8, 3.4, and 3.6, respectively) may meet the above criterion. However, the differences between the LD50 values of clinical and commensal E. cecorum strains were lower than those reported for virulent and avirulent S. Gallinarum43.
So far, only a single study has described gross lesions, while none has focused on histopathological lesions induced by E. cecorum in embryos25. The high initial EMR caused by the CB and CL E. cecorum strains coincided with significant pathomorphological lesions in dead embryos within 2 days. The severity of the pathomorphological lesions was found to be dependent not only on dose (bacterial concentration), but also on the isolate type. Embryos inoculated with the highest dose of clinical chicken (CB, BB, CL) E. cecorum isolates showed severe gross lesions involving mainly skeletal muscles, heart, yolk sac, stomach, liver, spleen, and kidneys. Besides the congestion of embryos, we found scattered petechial hemorrhages, usually on the skin (head, legs), organs (heart, gizzard), and mucous membranes (proventriculus). Congested dead embryos with prominent cranial and skin hemorrhages have also been recorded upon infection with E. coli (APEC) and Brucella microti21,39,40,44. Similarly, other authors observed ecchymotic hemorrhages and subcutaneous edema typical of sepsis in embryos inoculated with spinal E. cecorum isolates from broilers25, different Enterococcus species (E. faecalis, E. faecium, E. gallinarum) from meat turkeys24, and Mycoplasma spp.38,45. Based on the results obtained herein and in a previous study, all pathomorphological lesions can be associated with the generalized infection (bacteraemia). Contrary to the previous report25, minor abnormalities could be found in some embryos infected with a high concentration of commensal E. cecorum strains. Furthermore, some survivors exhibited pathomorphological lesions in organs; however, they showed neither dwarfism nor curled toes observed in embryos surviving infection with Mycoplasma lipofaciens45.
In histopathology, the liver, kidneys, heart, and brain were most affected by E. cecorum in dead and surviving embryos. Similarly, microscopic lesions in the heart, brain, and liver were found in embryos inoculated by APEC isolates, but most APEC-induced lesions occurred within 4 days. We found that E. cecorum could cause encephalomalacia just as APEC39. In contrast to E. cecorum, M. lipofaciens in another study did not affect the hearts of the embryos45. Histopathology provided the evidence of infiltration of heterophils and mononuclear cells in many organs of embryos that survived infection with E. cecorum (at a dose of approximately 3.4 × 107/egg) until the end of the study (7 dpi, 18 di), which means that E. cecorum can induce the host innate immune response by triggering an inflammatory response. Furthermore, reisolation of viable E. cecorum cells from these embryos indicated that E. cecorum might survive in tissues affected by the inflammatory process.
Our results showed that E. cecorum had a wide tissue distribution. High bacterial loads in heart samples may be due to the bacteraemia and special predilection of E. cecorum to the heart. Previous findings have indicated that bacteraemia and intestinal colonization due to pathogenic E. cecorum within the first 3 weeks of life are crucial to the pathogenesis of enterococcal spondylitis (ES) in broilers9. In this study, we showed that E. cecorum effectively replicated in chicken embryos. The number of reisolated E. cecorum from the whole hearts of dead embryos upon infection with clinical and commensal strains was higher than the number of bacteria inoculated. Moreover, bacterial loads were lower for clinical than for commensal isolates. This finding, together with the LD50 values, clearly indicated the pathogenicity of E. cecorum clinical strains. Our results revealed that bacterial loads of 6.57 × 102 cfu/heart for clinical E. cecorum and 18.3 × 102 cfu/heart for commensal E. cecorum were not mortal for some of 18-day-old chicken embryos. Moreover, the bacterial loads in the whole heart of surviving embryos did not exceed the LD50 values determined in this study for clinical and commensal strains, respectively. Although the study was terminated on the 18th day of incubation, we suppose that survivors could probably hatch despite the infection, which may suggest the possibility of transovarian transmission. It is worth mentioning that no strain was recovered from the control (non-infected) embryos, indicating that E. cecorum had no airborne dispersion in this study.
For the first time, the impact of E. cecorum on the body mass of infected embryos was evaluated. We found that the amount of bacteria in the inoculating dose did not influence the body mass of surviving embryos. However, body mass was significantly lower in the surviving embryos infected with clinical strains than in those infected with commensal strains. It implies that the pathogenicity status of E. cecorum strains (clinical vs. commensal), as well as classification to the poultry group strains (CB, BB, CL, T, W), have significant impact on the body mass of surviving embryos. Previous reports have indicated that inoculation of avian pathogenic E. faecalis via egg albumen or air-chamber may result in the growth depression of birds, whereas egg-dipping does not affect the weights41. According to Montgomery et al.46, chicks that hatched from E. coli-inoculated embryos revealed increased early mortality, decreased weight gains, and prolonged yolk sac absorption. In our study, infection with clinical E. cecorum strains from CB resulted in the lowest body mass of surviving embryos, which may adversely affect the hatch process or chick quality and growth performance.
Further research is needed to investigate the E. cecorum replication dynamics and rates in hatched chicks in order to gain a better understanding of bacterial physiology during infection.
Conclusions
In conclusion, the SPF chicken-embryo model used in this study confirmed the virulence of clinical E. cecorum from field outbreaks in 5 different poultry groups (CB, BB, CL, T, W) compared with commensal isolates. Our results indicated the highest pathogenicity of clinical E. cecorum isolates from CB and CL. Infection with CB and CL E. cecorum isolates resulted in the highest embryo mortality, shortest survival, lowest LD50 and severe pathomorphological lesions.
The majority of deaths caused by clinical or commensal E. cecorum occurred on 1 dpi. The exception are the CB and CL isolates which caused most deaths on 2 dpi. The deaths resulted from multiple organ damage due to generalized infection. Given that lethality subsided after 2 dpi but that all of the embryos were still infected, those embryos were likely to die or were sickly runts. This may be true also for the commensals. Our data indicated that some SPF embryos could survive infection despite the same inoculating dose and isolate type. The embryos that did not die over the course of the 5-day ELA were still infected and heavily colonized even by the commensals.
For the first time, the LD50 values, histopathological lesions, impact on the body mass and tissue colonization following E. cecorum infection of embryos have been evaluated. This study provides the first results on the multiplication of E. cecorum in chicken embryos. The body mass of surviving embryos is isolate type- rather than dose-dependent. Furthermore, surviving embryos are able to elicit innate immune response to E. cecorum infection. At the same time, reisolation of viable bacteria from surviving embryos may suggest that E. cecorum evade or resist immune mechanisms in order to successfully persist in organs.
Although ELA revealed significant differences between commensal and clinical (all clinical, all chicken, CB, CL) E. cecorum isolates, we suggest that it may not be a valid pathogenicity assay because of the possible variability in some clinical isolates around the triggering of a cytokine cascade in the immature embryo that results in death. However, experimental studies are needed to compare the ability of disease induction by isolates from different poultry groups with high and low EMR. Further studies are also needed to analyse the early host immune response to E. cecorum and to determine the correlations between isolate properties and results in ELA.
Data availability
The data generated and analysed in this study are included in this published article (and its Supplementary Information files). Other datasets are available from the corresponding author on a reasonable request.
References
Devriese, L. A., Dutta, G. N., Farrow, J. A. E., Van De Kerckhove, A. & Phillips, B. A. Streptococcus cecorum, a New Species Isolated from Chickens. Int. J. Syst. Bacteriol. 33, 772–776 (1983).
Dolka, B., Chrobak-Chmiel, D., Makrai, L. & Szeleszczuk, P. Phenotypic and genotypic characterization of Enterococcus cecorum strains associated with infections in poultry. BMC Vet Res 12, 129 (2016).
Herdt, P. D. et al. Enterococcus cecorum osteomyelitis and arthritis in broiler chickens. Vlaams Diergeneeskundig Tijdschrift 5 (2008).
Stalker, M. J., Brash, M. L., Weisz, A., Ouckama, R. M. & Slavic, D. Arthritis and Osteomyelitis Associated with Enterococcus Cecorum Infection in Broiler and Broiler Breeder Chickens in Ontario, Canada. J. VET Diagn. Invest. 22, 643–645 (2010).
Borst, L. B. et al. Molecular epidemiology of Enterococcus cecorum isolates recovered from enterococcal spondylitis outbreaks in the southeastern United States. Avian Pathol. 41, 479–485 (2012).
Robbins, K. M. et al. An Outbreak and Source Investigation of Enterococcal Spondylitis in Broilers Caused by Enterococcus cecorum. Avian Dis. 56, 768–773 (2012).
Szeleszczuk, P., Dolka, B., Żbikowski, A., Dolka, I. & Peryga, M. Pierwszy przypadek enterokokowego zapalenia stawów kręgosłupa u kurcząt brojlerów w Polsce. Medycyna Weterynaryjna 69, 298–303 (2013).
Jung, A. & Rautenschlein, S. Comprehensive report of an Enterococcus cecorum infection in a broiler flock in Northern Germany. BMC Vet. Res. 10, 311 (2014).
Borst, L. B. et al. Pathogenesis of enterococcal spondylitis caused by Enterococcus cecorum in broiler chickens. Vet. Pathol. 54, 61–73 (2017).
EFSA Panel on Animal Health and Welfare (AHAW) et al. Assessment of listing and categorisation of animal diseases within the framework of the Animal Health Law (Regulation (EU) No 2016/429): antimicrobial‐resistant Enterococcus cecorum in poultry. EFS2 20, (2022).
Jung, A., Metzner, M., Köhler-Repp, D. & Rautenschlein, S. Experimental reproduction of an Enterococcus cecorum infection in Pekin ducks. Avian Pathol. 42, 552–556 (2013).
Dolka, B., Chrobak-Chmiel, D., Czopowicz, M. & Szeleszczuk, P. Characterization of pathogenic Enterococcus cecorum from different poultry groups: Broiler chickens, layers, turkeys, and waterfowl. PLoS ONE 12, e0185199 (2017).
Jung, A., Metzner, M. & Ryll, M. Comparison of pathogenic and non-pathogenic Enterococcus cecorum strains from different animal species. BMC Microbiol. 17, 33 (2017).
Makrai, L. et al. Association of Enterococcus cecorum with vertebral osteomyelitis and spondylolisthesis in broiler parent chicks. Acta Vet. Hung. 59, 11–21 (2011).
Jung, A., Teske, L. & Rautenschlein, S. Enterococcus cecorum infection in a racing pigeon. Avian Dis. 58, 654–658 (2014).
Stępień-Pyśniak, D. et al. Prevalence and antibiotic resistance of Enterococcus strains isolated from poultry. Acta Vet. Hung. 64, 148–163 (2016).
Boerlin, P. et al. Diversity of Enterococcus cecorum from chickens. Vet. Microbiol. 157, 405–411 (2012).
Kense, M. J. & Landman, W. J. M. Enterococcus cecorum infections in broiler breeders and their offspring: molecular epidemiology. Avian Pathol. 40, 603–612 (2011).
Jackson, C. R. et al. Antimicrobial resistance, virulence determinants and genetic profiles of clinical and nonclinical Enterococcus cecorum from poultry. Lett. Appl. Microbiol. 60, 111–119 (2015).
Borst, L. B., Suyemoto, M. M., Scholl, E. H., Fuller, F. J. & Barnes, H. J. Comparative genomic analysis identifies divergent genomic features of pathogenic Enterococcus cecorum including a Type IC CRISPR-Cas System, a capsule locus, an epa-like locus, and putative host tissue binding proteins. PLoS ONE 10, e0121294 (2015).
Wareth, G. et al. Experimental infection of chicken embryos with recently described Brucella microti: Pathogenicity and pathological findings. Comp. Immunol. Microbiol. Infect. Dis. 41, 28–34 (2015).
Blanco, A. E. et al. Chicken embryo lethality assay for determining the lethal dose and virulence of Enterococcus faecalis. Avian Pathol. 46, 548–555 (2017).
Ozaki, H., Yonehara, K. & Murase, T. Virulence of Escherichia coli isolates obtained from layer chickens with colibacillosis associated with pericarditis, perihepatitis, and salpingitis in experimentally infected chicks and embryonated eggs. Avian Dis. 62, 233–236 (2018).
Maasjost, J., Lüschow, D., Kleine, A., Hafez, H. M. & Mühldorfer, K. Presence of virulence genes in Enterococcus species isolated from meat Turkeys in Germany does not correlate with chicken embryo lethality. Biomed. Res. Int. 2019, 1–10 (2019).
Borst, L. B. et al. A chicken embryo lethality assay for pathogenic Enterococcus cecorum. Avian Dis. 58, 244–248 (2014).
Ekesi, N. S., Hasan, A., Parveen, A., Shwani, A. & Rhoads, D. D. Embryo lethality assay as a tool for assessing virulence of isolates from bacterial chondronecrosis with osteomyelitis in broilers. Poultr. Sci. 100,101455. https://doi.org/10.1016/j.psj.2021.101455 (2021).
Dupont, H., Montravers, P., Mohler, J. & Carbon, C. Disparate findings on the role of virulence factors of Enterococcus faecalis in mouse and rat models of peritonitis. Infect. Immun. 66, 2570–2575 (1998).
Chen, S. et al. Metschnikowia bicuspidata and Enterococcus faecium co-infection in the giant freshwater prawn Macrobrachium rosenbergii. Dis. Aquat. Org. 55, 161–167 (2003).
Blanco, A. E. et al. Characterization of Enterococcus faecalis isolates by chicken embryo lethality assay and ERIC-PCR. Avian Pathol. 47, 23–32 (2018).
Rizkiantino, R. et al. Experimental infection of Enterococcus faecalis in Red Tilapia (Oreochromis hybrid) revealed low pathogenicity to cause streptococcosis. Open Vet. J. 11, 309 (2021).
Andersson, C., Gripenland, J. & Johansson, J. Using the chicken embryo to assess virulence of Listeria monocytogenes and to model other microbial infections. Nat. Protoc. 10, 1155–1164 (2015).
Reed, L. J. & Muench, H. A simple method of estimating fifty per cent endpoints. Am. J. Epidemiol. 27, 493–497 (1938).
Ramakrishnan, M. A. Determination of 50% endpoint titer using a simple formula. WJV 5, 85 (2016).
Jackson, C. R., Fedorka-Cray, P. J. & Barrett, J. B. Use of a genus- and species-specific multiplex PCR for identification of enterococci. J. Clin. Microbiol. 42, 3558–3565 (2004).
Markowski, E. P. & Markowski, C. A. A systematic method for teaching post hoc analysis of chi-square tests. Decis. Sci. J. Innov. Educ. 7, 59–65 (2009).
Gibbs, P. S. & Wooley, R. E. Comparison of the intravenous chicken challenge method with the embryo lethality assay for studies in avian colibacillosis. Avian Dis. 47, 672–680 (2003).
Seo, H.-S., Cha, S.-Y., Kang, M. & Jang, H.-K. Chicken embryo lethality assay for determining the virulence of Riemerella anatipestifer isolates. Avian Pathol. 42, 387–392 (2013).
Akhtar, M., Nazli, A. & Khan, M. A. chick embryo mortality studies using different strains of Mycoplasma Gallisepticum. Med. J. Islamic World Acad. Sci. 4, 297–300 (1991).
Wooley, R. E., Gibbs, P. S., Brown, T. P. & Maurer, J. J. Chicken embryo lethality assay for determining the virulence of avian Escherichia coli isolates. Avian Dis. 44, 318 (2000).
Rezaee, M. S., Liebhart, D., Hess, C., Hess, M. & Paudel, S. Bacterial infection in chicken embryos and consequences of yolk sac constitution for embryo survival. Vet. Pathol. 58, 71–79 (2021).
Landman, W. J. M., Mekkes, D. R., Chamanza, R., Doornenbal, P. & Gruys, E. Arthropathic and amyloidogenic Enterococcus faecalis infections in brown layers: a study on infection routes. Avian Pathol. 28, 545–557 (1999).
Lockaby, S. B., Hoerr, F. J., Kleven, S. H. & Lauerman, L. H. Pathogenicity of Mycoplasma synoviae in chicken embryos. Avian Dis. 43, 331–337 (1999).
Zhang, J. et al. The use of embryonic chicken eggs as an alternative model to evaluate the virulence of Salmonella enterica serovar Gallinarum. PLoS ONE 15, e0238630 (2020).
Adrenalin, S. L. et al. Virulence characteristic of avian pathogenic Escherichia coli (APEC) isolates. Jurnal Sain Veteriner. 38, 61 (2020).
Lierz, M., Stark, R., Brokat, S. & Hafez, H. M. Pathogenicity of Mycoplasma lipofaciens strain ML64, isolated from an egg of a Northern Goshawk (Accipiter gentilis), for chicken embryos. Avian Pathol. 36, 151–153 (2007).
Montgomery, R. D., Boyle, C. R., Lenarduzzi, T. A. & Jones, L. S. Consequences to chicks hatched from Escherichia coli-inoculated embryos. Avian Dis. 43, 553–563 (1999).
Funding
This work was supported by the National Science Centre (NCN), Poland under decision number DEC-2017/01/X/NZ7/00820.
Author information
Authors and Affiliations
Contributions
B.D. designed the study, obtained funds for research, performed the experiment, analysed the data, interpreted the results and wrote the manuscript. M.C. performed statistical analysis, contributed to result interpretation. I.D. performed histopathological examination, contributed to result interpretation. B.D., M.C., I.D., P.S. contributed to the writing of the manuscript draft. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dolka, B., Czopowicz, M., Dolka, I. et al. Chicken embryo lethality assay for determining the lethal dose, tissue distribution and pathogenicity of clinical Enterococcus cecorum isolates from poultry. Sci Rep 12, 10675 (2022). https://doi.org/10.1038/s41598-022-14900-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-022-14900-9
This article is cited by
Comments
By submitting a comment you agree to abide by our Terms and Community Guidelines. If you find something abusive or that does not comply with our terms or guidelines please flag it as inappropriate.