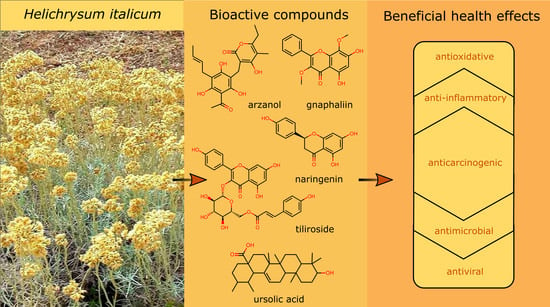
Helichrysum italicum: From Extraction, Distillation, and Encapsulation Techniques to Beneficial Health Effects
Abstract
:1. Introduction
Identification and Taxonomic Classification of Helichrysum italicum
- (1)
- subsp. italicum (Corsica, Italy, Cyprus, isolated localities in Morocco)
- (2)
- subsp. microphyllum (Willd.) Nyman (Balearic Islands, Sardinia, Corsica, Crete, and Cyprus)
- (3)
- subsp. picardii (France, Italy, Portugal, and Spain)
- (4)
- subsp. pseudolitoreum (Argentario, Gargano, and Mount Conero)
- (5)
- subsp. serotinum (Iberian Peninsula)
- (6)
- subsp. siculum (Sicily) [5].
- (1)
- subsp. italicum (Italy, Croatia, eastern Mediterranean coast of France and Corsica, Bosnia and Herzegovina, Greece -Aegean islands and Cyprus),
- (2)
- subsp. microphyllum (Crete),
- (3)
- subsp. siculum (Sicily), and
- (4)
- subsp. tyrrhenicum (Corsica, Sardinia, Majorca, and Dragonera Islet).
2. Extraction, Distillation, and Analytical Methods for Obtaining Extracts, Essential Oils as Well as Individual Bioactive Compounds from Helichrysum italicum
| Compound Class | Compounds | Isolation Techniques | Identification Methods | References |
|---|---|---|---|---|
| Essential oils | ||||
| Monoterpenes | α-pinene, limonene, nerol, neryl acetate, and neryl propanoate | hydrodistillation with Clevenger-type apparatus | GC-FID, GC-MS | [15,26,27,33,34,35,36,37,38,39,40,41,42,43] |
| steam distillation with spring-type apparatus | GC-FID, GC-MS | [4,6] | ||
| Sesquiterpenes | α–selinene, β-selinene, γ-curcumene, and eudesm-5-en-11-ol | hydrodistillation with Clevenger-type apparatus | GC-FID, GC-MS | [40,44,45,46,47,48] |
| steam distillation with spring-type apparatus | GC-FID, GC-MS | [4] | ||
| Extracts | ||||
| Polyphenolic acids | chlorogenic acid, caffeic acid | accelerated solvent extraction using methanol-water (3:1) | HPLC-MS/MS | [49] |
| solvent extraction using methanol | HPLC, HRESIMS/MS, 1H NMR, 13C NMR, DQF-COSY | [50] | ||
| solvent extraction using methanol | HPLC-DAD | [39] | ||
| solvent extraction using ethanol | UV-VIS, IR, MS | [51] | ||
| HPLC, 1H NMR | [52] | |||
| Flavonoids | gnaphalin, tiliroside, pinocembrin | solvent extraction using methanol | Gravity column chromatography on silica gel, UV, IR, 1H NMR, 13C NMR, HRESIMS | [21,53,54] |
| naringenin, kaempferol, quercetin | accelerated solvent extraction using methanol-water (3:1) | HPLC-MS/MS | [49] | |
| solvent extraction using methanol | HPLC, HRESIMS, 1H NMR, 13C NMR, DQF-COSY | [50] | ||
| gnaphalin, naringenin, apigenin, luteolin, kaempferol, quercetin | solvent extraction using ethanol (70%) | UV-VIS, EI-MS, FD-MS | [3] | |
| HPLC, UV-VIS | [55] | |||
| MECC-DAD, HPLC-DAD, UV-VIS | [56] | |||
| Pyrones | arzanol | solvent extraction using acetone | Gravity column chromatography on silica gel, HPLC, HRESIMS, 1H NMR, 13C NMR, IR, UV | [10,12,13] |
| Acetophenones | 4-hydroxy-3-(2-hydroxy-3-isopentenyl)acetophenone 4-hydroxy-3-(3-methyl-2-butenyl) acetophenone | solvent extraction with methanol | gravity column chromatography on silica gel, UV, IR, 1H NMR, 13C NMR, HRESIMS | [53] |
| TLC, HPLC-DAD | [21] | |||
| Tremetones | 12-acetoxytremetone | solvent extraction with ethanol | Gravity column chromatography on silica gel, HPLC, ESI-MS, UV, IR, 1H NMR, 13C NMR, DQF-COSY | [57,58] |
| solvent extraction with acetone | Gravity column chromatography on silica gel, HRESIMS, 1H NMR, 13C NMR, IR, UV | [10,12] | ||
| 12-hydroxytremetone | solvent extraction with methanol | Gravity column chromatography on silica gel, UV, IR, 1H NMR, 13C NMR, HRESIMS | [53] | |
| Triterpenes | ursolic acid | solvent extraction with methanol | Gravity column chromatography on silica gel, UV, IR, 1H NMR, 13C NMR, HRESIMS | [53] |
| TLC, HPLC-DAD | [21] | |||
| solvent extraction with acetone | Gravity column chromatography on silica gel, HPLC, HRESIMS, 1H NMR, 13C NMR | [13] | ||
3. Methods and Techniques for Determining Biological Effects of Extracts, Essential Oils as Well as Individual Bioactive Compounds from Helichrysum italicum
3.1. Methods and Techniques for Determining Antioxidative Effects
3.2. Methods and Techniques for Determining Antimicrobial Effects
3.3. Methods and Techniques for Determining Anticarcinogenic Effects
3.4. Methods and Techniques for Determining Anti-Inflammatory Effects
4. Biological Effects of Helichrysum italicum Extracts
4.1. Biological Effects of Major Bioactive Compounds from Helichrysum italicum Extracts
4.1.1. Phenolic Acids
4.1.2. Flavonoids
4.1.3. Acetophenones and Tremetones
4.1.4. Pyrones
4.1.5. Triterpenes
5. Biological Effects of Helichrysum italicum Essential Oils
5.1. Biological Effects of Major Bioactive Compounds from Helichrysum italicum Essential Oils
5.1.1. Monoterpenes
5.1.2. Sesquiterpenes
6. Encapsulation of Helichrysum italicum Extracts, Essential Oils and Individual Bioactive Compounds
7. Conclusions and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| 5-LOX | 5-lipoxygenase |
| ABTS | 2,2’-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) |
| AChE | Acetylcholinesterase |
| AD | Alzheimer’s disease |
| CI | Confidence interval |
| COSY | Correlated spectroscopy |
| COX1 | Cyclooxygenase 1 |
| COX2 | Cyclooxygenase 2 |
| CYP | cytochrome P450 enzymes |
| DAD | Diode array detection |
| DNA | Deoxyribonucleic acid |
| DPPH | 2,2-diphenyl-1-picrylhydrazyl |
| EC50 | Half maximal effective concentration |
| EI-MS | Electron ionization mass spectrometry |
| EO | Essential oil |
| FID | Flame ionization detector |
| GC | Gas chromatography |
| GC-MS | Gas chromatography-mass spectrometry |
| GI | Growth inhibition |
| HDAC | Histone deacetylases |
| HIV | Human immunodeficiency virus |
| HPLC | High performance liquid chromatography |
| HRESIMS | High-resolution electrospray ionisation mass spectrometry |
| HSV | Herpes simplex virus |
| IC50 | The half maximal inhibitory concentration |
| ID50 | Infectious dose 50 |
| IL-1β | Interleukin-1beta |
| IL-6 | Interleukin-6 |
| IL-8 | Interleukin-8 |
| IL-12 | Interleukin-12 subunit p40 |
| LC50 | Median lethal concentration |
| LD50 | Median lethal dose |
| LDL | human low density lipoprotein |
| LPS | Lipopolysaccharide |
| LTB4 | leukotriene B4 |
| ITS1/2 | Internal transcribed spacer 1 and 2 |
| MBC | Minimum bactericidal concentration |
| MECC | Micellar electrokinetic capillary chromatography |
| MIC | Minimum inhibitory concentration |
| mRNA | Messenger ribonucleic acid |
| mPGES | Microsomal PEG2 synthase |
| MTS | 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium |
| MTT | 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| NF-κB | Nuclear factor kappa B |
| NMR | Nuclear magnetic resonance |
| NO | Nitric oxide |
| PTP1B | Protein tyrosine phosphatase 1B |
| PGE2 | Prostaglandin E2 |
| PGF1α | Prostaglandin F1 alpha |
| PLA2 | Phospholipase A2 |
| SFE | Supercritical fluid extraction |
| sub-MIC | Sub-minimum inhibitory concentration |
| TBH | Tert-butyl hydroperoxide |
| TLC | Thin-layer chromatography |
| TNF-α | Tumor necrosis factor alpha |
| TPA | 12-O-Tetradecanoylphorbol-13-acetate |
| TRPA1 | Transient receptor potential cation channel, subfamily A, member 1 |
| UV-VIS | Ultraviolet–visible spectroscopy |
| VSV | Vesicular stomatitis virus |
References
- Iriti, M.; Varoni, E.M.; Vitalini, S. Melatonin in traditional Mediterranean diets. J. Pineal Res. 2010, 49, 101–105. [Google Scholar] [CrossRef] [PubMed]
- Tira, S.; Di Modica, G.; Rossi, P. Isolamento e riconoscimento di acidi presenti in Helichrysum italicum G. Don. Atti Dell’academia Sci. Fis. 1959, 94, 185–190. [Google Scholar]
- Maffei Facino, R.; Carini, M.; Mariani, M.; Cipriani, C. Anti-erythematous and photoprotective activities in guinea pigs and man of topically applied flavonoids from Helichrysum italicum G. Don. Acta Ther. 1988, 14, 323–345. [Google Scholar]
- Morone-Fortunato, I.; Montemurro, C.; Ruta, C.; Perrini, R.; Sabetta, W.; Blanco, A.; Lorusso, E.; Avato, P. Essential oils, genetic relationships and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. italicum from wild Mediterranean germplasm. Ind. Crops Prod. 2010, 32, 639–649. [Google Scholar] [CrossRef]
- Viegas, D.A.; Palmeira-de-Oliveira, A.; Salgueiro, L.; Martinez-de-Oliveira, J.; Palmeira-de-Oliveira, R. Helichrysum italicum: From traditional use to scientific data. J. Ethnopharmacol. 2014, 151, 54–65. [Google Scholar] [CrossRef]
- Perrini, R.; Morone-Fortunato, I.; Lorusso, E.; Avato, P. Glands, essential oils and in vitro establishment of Helichrysum italicum (Roth) G. Don ssp. microphyllum (Willd.) Nyman. Ind. Crops Prod. 2009, 29, 395–403. [Google Scholar] [CrossRef]
- Peris, J.B.; Stubing, G.; Romo, A. Plantas Medicinales de la Península Ibérica e Islas Baleares; Jaguar: Madrid, Spain, 2001. [Google Scholar]
- Goodfriend, C. Aromatherapy for pregnancy and birth. Int. J. Childbirth Educ. 2001, 16, 18. [Google Scholar]
- Voinchet, V.; Giraud-Robert, A.-M. Utilisation de l’huile essentielle d’hélichryse italienne et de l’huile végétale de rose musquée après intervention de chirurgie plastique réparatrice et esthétique. Phytothérapie 2007, 5, 67–72. [Google Scholar] [CrossRef]
- Appendino, G.; Ottino, M.; Marquez, N.; Bianchi, F.; Giana, A.; Ballero, M.; Sterner, O.; Fiebich, B.L.; Munoz, E. Arzanol, an anti-inflammatory and anti-HIV-1 phloroglucinol α-pyrone from Helichrysum italicum ssp. microphyllum. J. Nat. Prod. 2007, 70, 608–612. [Google Scholar] [CrossRef]
- Bauer, J.; Koeberle, A.; Dehm, F.; Pollastro, F.; Appendino, G.; Northoff, H.; Rossi, A.; Sautebin, L.; Werz, O. Arzanol, a prenylated heterodimeric phloroglucinyl pyrone, inhibits eicosanoid biosynthesis and exhibits anti-inflammatory efficacy in vivo. Biochem. Pharmacol. 2011, 81, 259–268. [Google Scholar] [CrossRef]
- Rosa, A.; Deiana, M.; Atzeri, A.; Corona, G.; Incani, A.; Melis, M.P.; Appendino, G.; Dessì, M.A. Evaluation of the antioxidant and cytotoxic activity of arzanol, a prenylated α-pyrone–phloroglucinol etherodimer from Helichrysum italicum subsp. microphyllum. Chem. Biol. Interact. 2007, 165, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Taglialatela-Scafati, O.; Pollastro, F.; Chianese, G.; Minassi, A.; Gibbons, S.; Arunotayanun, W.; Mabebie, B.; Ballero, M.; Appendino, G. Antimicrobial phenolics and unusual glycerides from Helichrysum italicum subsp. microphyllum. J. Nat. Prod. 2012, 76, 346–353. [Google Scholar] [CrossRef] [PubMed]
- Djihane, B.; Wafa, N.; Elkhamssa, S.; Maria, A.E.; Mihoub, Z.M. Chemical constituents of Helichrysum italicum (Roth) G. Don essential oil and their antimicrobial activity against Gram-positive and Gram-negative bacteria, filamentous fungi and Candida albicans. Saudi Pharm. J. 2016, 25, 780–787. [Google Scholar] [CrossRef] [PubMed]
- Conti, B.; Canale, A.; Bertoli, A.; Gozzini, F.; Pistelli, L. Essential oil composition and larvicidal activity of six Mediterranean aromatic plants against the mosquito Aedes albopictus (Diptera: Culicidae). Parasitol. Res. 2010, 107, 1455–1461. [Google Scholar] [CrossRef] [PubMed]
- Petelin, A.; Šik Novak, K.; Hladnik, M.; Bandelj, D.; Baruca Arbeiter, A.; Kramberger, K.; Kenig, S.; Jenko Pražnikar, Z. Helichrysum italicum (Roth) G. Don and Helichrysum arenarium (L.) Moench Infusion Consumption Affects the Inflammatory Status and the Composition of Human Gut Microbiota in Patients with Traits of Metabolic Syndrome: A Randomized Comparative Study. Foods 2022, 11, 3277. [Google Scholar] [CrossRef]
- Tundis, R.; Statti, G.; Conforti, F.; Bianchi, A.; Agrimonti, C.; Sacchetti, G.; Muzzoli, M.; Ballero, M.; Menichini, F.; Poli, F. Influence of environmental factors on composition of volatile constituents and biological activity of Helichrysum italicum (Roth) Don (Asteraceae). Nat. Prod. Res. 2005, 19, 379–387. [Google Scholar] [CrossRef]
- Ninčević, T.; Grdiša, M.; Šatović, Z.; Jug-Dujaković, M. Helichrysum italicum (Roth) G. Don: Taxonomy, biological activity, biochemical and genetic diversity. Ind. Crops Prod. 2019, 138, 111487. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.d.C.; Giner, R.M.; Máñez, S.; Tournier, H.; Schinella, G.; Ríos, J.L. Anti-inflammatory and antioxidant properties of Helichrysum italicum. J. Pharm. Pharmacol. 2002, 54, 365–371. [Google Scholar] [CrossRef]
- Nostro, A.; Bisignano, G.; Cannatelli, M.A.; Crisafi, G.; Germano, M.P.; Alonzo, V. Effects of Helichrysum italicum extract on growth and enzymatic activity of Staphylococcus aureus. Int. J. Antimicrob. Agents 2001, 17, 517–520. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Cerdá-Nicolás, M.; Ríos, J.-L. Assessment of the anti-inflammatory activity and free radical scavenger activity of tiliroside. Eur. J. Pharmacol. 2003, 461, 53–61. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.; Marino, A.; Picerno, I.; Pizzimenti, F.; Scoglio, M.; Spataro, P. Evaluation of antiherpesvirus-1 and genotoxic activities of Helichrysum italicum extract. New Microbiol. 2003, 26, 125–128. [Google Scholar]
- Guinoiseau, E.; Lorenzi, V.; Luciani, A.; Muselli, A.; Costa, J.; Casanova, J.; Berti, L. Biological properties and resistance reversal effect of Helichrysum italicum (Roth) G. Don. Microb. Pathog. Strateg. Combat. Sci. Technol. Educ. 2013, 2, 1073–1080. [Google Scholar]
- Herrando Moraira, S.; Blanco Moreno, J.M.; Sáez, L.; Galbany Casals, M. Re-evaluation of Helichrysum italicum complex (Compositae: Gnaphalieae): A new species from Majorca (Balearic Islands). Collect. Bot. 2016, 35, 009. [Google Scholar]
- Bouchaala, M.; Ramdani, M.; Lograda, T.; Chalard, P.; Figueredo, G. Chemical Composition, Antibacterial Activity and Chromosome Number of Helichrysum lacteum, Endemic from Algeria. Int. J. Pharma. Res. Health Sci. 2017, 5, 1539–1545. [Google Scholar]
- Melito, S.; Sias, A.; Petretto, G.L.; Chessa, M.; Pintore, G.; Porceddu, A. Genetic and metabolite diversity of Sardinian populations of Helichrysum italicum. PLoS ONE 2013, 8, e79043. [Google Scholar] [CrossRef]
- Melito, S.; Petretto, G.; Podani, J.; Foddai, M.; Maldini, M.; Chessa, M.; Pintore, G. Altitude and climate influence Helichrysum italicum subsp. microphyllum essential oils composition. Ind. Crops Prod. 2016, 80, 242–250. [Google Scholar] [CrossRef]
- Giovino, A.; Martinelli, F.; Perrone, A. The technique of Plant DNA Barcoding: Potential application in floriculture. Caryologia 2020, 73, 27–38. [Google Scholar]
- De Mattia, F.; Bruni, I.; Galimberti, A.; Cattaneo, F.; Casiraghi, M.; Labra, M. A comparative study of different DNA barcoding markers for the identification of some members of Lamiacaea. Food Res. Int. 2011, 44, 693–702. [Google Scholar] [CrossRef]
- Ratnasingham, S.; Hebert, P.D. BOLD: The Barcode of Life Data System. Mol. Ecol. Notes 2007, 7, 355–364. Available online: http://www.barcodinglife.org (accessed on 9 February 2023). [CrossRef]
- Arbeiter, A.B.; Hladnik, M.; Jakše, J.; Bandelj, D. First set of microsatellite markers for immortelle (Helichrysum italicum (Roth) G. Don): A step towards the selection of the most promising genotypes for cultivation. Ind. Crops Prod. 2021, 162, 113298. [Google Scholar] [CrossRef]
- Maksimovic, S.; Tadic, V.; Skala, D.; Zizovic, I. Separation of phytochemicals from Helichrysum italicum: An analysis of different isolation techniques and biological activity of prepared extracts. Phytochemistry 2017, 138, 9–28. [Google Scholar] [CrossRef] [PubMed]
- Leonardi, M.; Ambryszewska, K.E.; Melai, B.; Flamini, G.; Cioni, P.L.; Parri, F.; Pistelli, L. Essential-Oil Composition of Helichrysum italicum (Roth) G. Don ssp. italicum from Elba Island (Tuscany, Italy). Chem. Biodivers. 2013, 10, 343–355. [Google Scholar] [CrossRef] [PubMed]
- Mastelic, J.; Politeo, O.; Jerkovic, I.; Radosevic, N. Composition and antimicrobial activity of Helichrysum italicum essential oil and its terpene and terpenoid fractions. Chem. Nat. Compd. 2005, 41, 35–40. [Google Scholar] [CrossRef]
- Mastelić, J.; Politeo, O.; Jerković, I. Contribution to the analysis of the essential oil of Helichrysum italicum (Roth) G. Don.–determination of ester bonded acids and phenols. Molecules 2008, 13, 795–803. [Google Scholar] [CrossRef] [PubMed]
- Ornano, L.; Venditti, A.; Sanna, C.; Ballero, M.; Maggi, F.; Lupidi, G.; Bramucci, M.; Quassinti, L.; Bianco, A. Chemical composition and biological activity of the essential oil from Helichrysum microphyllum Cambess. ssp. tyrrhenicum Bacch., Brullo e Giusso growing in La Maddalena Archipelago, Sardinia. J. Oleo Sci. 2015, 64, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Bertoli, A.; Conti, B.; Mazzoni, V.; Meini, L.; Pistelli, L. Volatile chemical composition and bioactivity of six essential oils against the stored food insect Sitophilus zeamais Motsch.(Coleoptera Dryophthoridae). Nat. Prod. Res. 2012, 26, 2063–2071. [Google Scholar]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Zhiri, A.; Idaomar, M. Cytotoxicity and gene induction by some essential oils in the yeast Saccharomyces cerevisiae. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2005, 585, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Węglarz, Z.; Kosakowska, O.; Pióro-Jabrucka, E.; Przybył, J.L.; Gniewosz, M.; Kraśniewska, K.; Szyndel, M.S.; Costa, R.; Bączek, K.B. Antioxidant and antibacterial activity of Helichrysum italicum (Roth) G. Don. from central Europe. Pharmaceuticals 2022, 15, 735. [Google Scholar] [CrossRef]
- Bianchini, A.; Santoni, F.; Paolini, J.; Bernardini, A.F.; Mouillot, D.; Costa, J. Partitioning the relative contributions of inorganic plant composition and soil characteristics to the quality of Helichrysum italicum subsp. italicum (Roth) G. Don fil. essential oil. Chem. Biodivers. 2009, 6, 1014–1033. [Google Scholar] [CrossRef]
- Kladar, N.V.; Anačkov, G.T.; Rat, M.M.; Srđenović, B.U.; Grujić, N.N.; Šefer, E.I.; Božin, B.N. Biochemical characterization of Helichrysum italicum (Roth) G. Don subsp. italicum (Asteraceae) from Montenegro: Phytochemical screening, chemotaxonomy, and antioxidant properties. Chem. Biodivers. 2015, 12, 419–431. [Google Scholar] [CrossRef]
- Cui, H.; Zhao, C.; Lin, L. Antibacterial Activity of H elichrysum italicum Oil on Vegetables and Its Mechanism of Action. J. Food Process. Preserv. 2015, 39, 2663–2672. [Google Scholar] [CrossRef]
- Cui, H.; Li, W.; Li, C.; Lin, L. Synergistic effect between Helichrysum italicum essential oil and cold nitrogen plasma against Staphylococcus aureus biofilms on different food-contact surfaces. Int. J. Food Sci. Tech. 2016, 51, 2493–2501. [Google Scholar] [CrossRef]
- Paolini, J.; Desjobert, J.M.; Costa, J.; Bernardini, A.F.; Castellini, C.B.; Cioni, P.L.; Flamini, G.; Morelli, I. Composition of essential oils of Helichrysum italicum (Roth) G. Don fil subsp. italicum from Tuscan archipelago islands. Flavour. Fragr. J. 2006, 21, 805–808. [Google Scholar] [CrossRef]
- Roussis, V.; Tsoukatou, M.; Petrakis, P.V.; Chinou, I.; Skoula, M.; Harborne, J.B. Volatile constituents of four Helichrysum species growing in Greece. Biochem. Syst. Ecol. 2000, 28, 163–175. [Google Scholar] [CrossRef]
- Maggio, A.; Bruno, M.; Guarino, R.; Senatore, F.; Ilardi, V. Contribution to a Taxonomic Revision of the Sicilian Helichrysum Taxa by PCA Analysis of Their Essential-Oil Compositions. Chem. Biodivers. 2016, 13, 151–159. [Google Scholar] [CrossRef]
- Schipilliti, L.; Bonaccorsi, I.L.; Ragusa, S.; Cotroneo, A.; Dugo, P. Helichrysum italicum (Roth) G. Don fil. subsp. italicum oil analysis by gas chromatography–carbon isotope ratio mass spectrometry (GC-C-IRMS): A rapid method of genotype differentiation? J. Essent. Oil Res. 2016, 28, 193–201. [Google Scholar] [CrossRef]
- Stupar, M.; Ljaljević-Grbić, M.; Džamić, A.; Unković, N.; Ristić, M.; Vukojević, J. Antifungal activity of Helichrysum italicum (Roth) G. Don (Asteraceae) essential oil against fungi isolated from cultural heritage objects. Arch. Biol. Sci. 2014, 66, 1539–1545. [Google Scholar] [CrossRef]
- de la Garza, A.L.; Etxeberria, U.; Lostao, M.a.P.; San Román, B.n.; Barrenetxe, J.; Martínez, J.A.; Milagro, F.n.I. Helichrysum and grapefruit extracts inhibit carbohydrate digestion and absorption, improving postprandial glucose levels and hyperinsulinemia in rats. J. Agric. Food Chem. 2013, 61, 12012–12019. [Google Scholar] [CrossRef]
- Mari, A.; Napolitano, A.; Masullo, M.; Pizza, C.; Piacente, S. Identification and quantitative determination of the polar constituents in Helichrysum italicum flowers and derived food supplements. J. Pharm. Biomed. Anal. 2014, 96, 249–255. [Google Scholar] [CrossRef]
- Zapesochnaya, G.; Dzyadevich, T.; Karasartov, B. Phenolic compounds of Helichrysum italicum. Chem. Nat. Compd. 1990, 26, 342–343. [Google Scholar] [CrossRef]
- Zapesochnaya, G.; Kurkin, V.; Kudryavtseva, T.; Karasartov, B.; Cholponbaev, K.; Tyukavkina, N.; Ruchkin, V. Dicaffeolyquinic acids from Helichrysum italicum and Achillea cartilaginea. Chem. Nat. Compd. 1992, 28, 40–44. [Google Scholar] [CrossRef]
- Sala, A.; Recio, M.d.C.; Giner, R.M.; Máñez, S.; Ríos, J.-L. New Acetophenone Glucosides Isolated from Extracts of Helichrysum italicum with Antiinflammatory Activity. J. Nat. Prod. 2001, 64, 1360–1362. [Google Scholar] [CrossRef]
- Schinella, G.R.; Tournier, H.A.; Máñez, S.; de Buschiazzo, P.M.; del Carmen Recio, M.; Ríos, J.L. Tiliroside and gnaphaliin inhibit human low density lipoprotein oxidation. Fitoterapia 2007, 78, 1–6. [Google Scholar] [CrossRef]
- Pietta, P.; Mauri, P.; Gardana, C.; Facino, R.M.; Carini, M. High-performance liquid chromatographic determination of flavonoid glucosides from Helichrysum italicum. J. Chromatogr. A 1991, 537, 449–452. [Google Scholar] [CrossRef]
- Pietta, P.; Mauri, P.; Facino, R.M.; Carini, M. Analysis of flavonoids by MECC with ultraviolet diode array detection. J. Pharm. Biomed. Anal. 1992, 10, 1041–1045. [Google Scholar] [CrossRef] [PubMed]
- Rigano, D.; Formisano, C.; Senatore, F.; Piacente, S.; Pagano, E.; Capasso, R.; Borrelli, F.; Izzo, A.A. Intestinal antispasmodic effects of Helichrysum italicum (Roth) Don ssp. italicum and chemical identification of the active ingredients. J. Ethnopharmacol. 2013, 150, 901–906. [Google Scholar] [CrossRef]
- Rigano, D.; Formisano, C.; Pagano, E.; Senatore, F.; Piacente, S.; Masullo, M.; Capasso, R.; Izzo, A.A.; Borrelli, F. A new acetophenone derivative from flowers of Helichrysum italicum (Roth) Don ssp. italicum. Fitoterapia 2014, 99, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Union, E. Directive 2009/28/EC of the European Parliament and of the Council of 23 April 2009 on the promotion of the use of energy from renewable sources and amending and subsequently repealing Directives 2001/77/EC and 2003/30/EC. Off. J. Eur. Uni. 2009, 5, 2009. [Google Scholar]
- Ivanovic, J.; Ristic, M.; Skala, D. Supercritical CO2 extraction of Helichrysum italicum: Influence of CO2 density and moisture content of plant material. J. Supercrit. Fluids 2011, 57, 129–136. [Google Scholar] [CrossRef]
- Jerković, I.; Rajić, M.; Marijanović, Z.; Bilić, M.; Jokić, S. Optimization of supercritical CO2 extraction of dried Helichrysum italicum flowers by response surface methodology: GC-MS profiles of the extracts and essential oil. Sep. Sci. Technol. 2016, 51, 2925–2931. [Google Scholar] [CrossRef]
- Maksimovic, S.; Kesic, Z.; Lukic, I.; Milovanovic, S.; Ristic, M.; Skala, D. Supercritical fluid extraction of curry flowers, sage leaves, and their mixture. J. Supercrit. Fluids 2013, 84, 1–12. [Google Scholar] [CrossRef]
- Marongiu, B.; Piras, A.; Desogus, E.; Porcedda, S.; Ballero, M. Analysis of the volatile concentrate of the leaves and flowers of Helichrysum italicum (Roth) Don ssp. microphyllum (Willd.) Nyman (Asteraceae) by supercritical fluid extraction and their essential oils. J. Essent. Oil Res. 2003, 15, 120–126. [Google Scholar] [CrossRef]
- Mićić, V.; Jotanović, M.J.; Lepojević, Ž.; Aleksić, V.; Pejović, B. Pressure influence to extraction system Helichrysum italicum–supercritical carbon dioxide. J. Eng. Process. Manag. 2009, 1, 26–31. [Google Scholar]
- Costa, P.; Loureiro, J.M.; Teixeira, M.A.; Rodrigues, A.E. Extraction of aromatic volatiles by hydrodistillation and supercritical fluid extraction with CO2 from Helichrysum italicum subsp. picardii growing in Portugal. Ind. Crops Prod. 2015, 77, 680–683. [Google Scholar] [CrossRef]
- Maksimovic, S.; Tadic, V.; Zvezdanovic, J.; Zizovic, I. Utilization of supercritical CO2 in bioactive principles isolation from Helichrysum italicum and their adsorption on selected fabrics. J. Supercrit. Fluids 2021, 171, 105197. [Google Scholar] [CrossRef]
- Jokić, S.; Rajić, M.; Bilić, B.; Molnar, M. Supercritical extraction of scopoletin from Helichrysum italicum (Roth) G. Don flowers. Phytochem. Anal. 2016, 27, 290–295. [Google Scholar] [CrossRef]
- Kedare, S.B.; Singh, R. Genesis and development of DPPH method of antioxidant assay. J. Food Sci. Technol. 2011, 48, 412–422. [Google Scholar] [CrossRef]
- Huang, D.; Ou, B.; Prior, R.L. The chemistry behind antioxidant capacity assays. J. Agric. Food Chem. 2005, 53, 1841–1856. [Google Scholar] [CrossRef]
- Rosa, A.; Atzeri, A.; Nieddu, M.; Appendino, G. New insights into the antioxidant activity and cytotoxicity of arzanol and effect of methylation on its biological properties. Chem. Phys. Lipids 2017, 205, 55–64. [Google Scholar] [CrossRef]
- Rosa, A.; Pollastro, F.; Atzeri, A.; Appendino, G.; Melis, M.P.; Deiana, M.; Incani, A.; Loru, D.; Dessì, M.A. Protective role of arzanol against lipid peroxidation in biological systems. Chem. Phys. Lipids 2011, 164, 24–32. [Google Scholar] [CrossRef]
- Janero, D.R. Malondialdehyde and thiobarbituric acid-reactivity as diagnostic indices of lipid peroxidation and peroxidative tissue injury. Free Radic. Biol. Med. 1990, 9, 515–540. [Google Scholar] [CrossRef] [PubMed]
- Siu, A.W.; Reiter, R.J.; To, C.H. The efficacy of vitamin E and melatonin as antioxidants against lipid peroxidation in rat retinal homogenates. J. Pineal Res. 1998, 24, 239–244. [Google Scholar] [CrossRef] [PubMed]
- Balouiri, M.; Sadiki, M.; Ibnsouda, S.K. Methods for in vitro evaluating antimicrobial activity: A review. J. Pharm. Anal. 2016, 6, 71–79. [Google Scholar] [CrossRef]
- Domínguez, R.; Muñoz, R.; Araiza, H. Sequential injection analysis system for electronic tongues modelling and calibration process. In Proceedings of the 2010 7th International Conference on Electrical Engineering Computing Science and Automatic Control, Tuxtla Gutierrez, Mexico, 8–10 September 2010; pp. 280–284. [Google Scholar]
- Madigan, M.T.; Martinko, J.M.; Dunlap, P.V.; Clark, D.P. Brock biology of microorganisms 12th edn. Int. Microbiol. 2008, 11, 65–73. [Google Scholar]
- Oliva, A.; Garzoli, S.; Sabatino, M.; Tadić, V.; Costantini, S.; Ragno, R.; Božović, M. Chemical composition and antimicrobial activity of essential oil of Helichrysum italicum (Roth) G. Don fil.(Asteraceae) from Montenegro. Nat. Prod. Res. 2020, 34, 445–448. [Google Scholar] [CrossRef]
- Staver, M.M.; Gobin, I.; Ratkaj, I.; Petrovic, M.; Vulinovic, A.; Dinarina-Sablic, M.; Broznic, D. In vitro antiproliferative and antimicrobial activity of the essential oil from the flowers and leaves of Helichrysum italicum (Roth) G. Don growing in central Dalmatia (Croatia). J. Essent. Oil-Bear. Plants 2018, 21, 77–91. [Google Scholar] [CrossRef]
- Marks, D.C.; Belov, L.; Davey, M.W.; Davey, R.A.; Kidman, A.D. The MTT cell viability assay for cytotoxicity testing in multidrug-resistant human leukemic cells. Leuk. Res. 1992, 16, 1165–1173. [Google Scholar] [CrossRef]
- Hercog, K.; Maisanaba, S.; Filipič, M.; Sollner-Dolenc, M.; Kač, L.; Žegura, B. Genotoxic activity of bisphenol A and its analogues bisphenol S, bisphenol F and bisphenol AF and their mixtures in human hepatocellular carcinoma (HepG2) cells. Sci. Total Environ. 2019, 687, 267–276. [Google Scholar] [CrossRef]
- Žegura, B.; Filipič, M. Application of in vitro comet assay for genotoxicity testing. In Optimization in Drug Discovery; Springer: Berlin/Heidelberg, Germany, 2004; pp. 301–313. [Google Scholar]
- Singh, N.P.; McCoy, M.T.; Tice, R.R.; Schneider, E.L. A simple technique for quantitation of low levels of DNA damage in individual cells. Exp. Cell Res. 1988, 175, 184–191. [Google Scholar] [CrossRef]
- Cemeli, E.; Baumgartner, A.; Anderson, D. Antioxidants and the Comet assay. Mutat. Res. Rev. Mutat. Res. 2009, 681, 51–67. [Google Scholar] [CrossRef]
- Hariram Nile, S.; Won Park, S. Optimized methods for in vitro and in vivo anti-inflammatory assays and its applications in herbal and synthetic drug analysis. Mini Rev. Med. Chem. 2013, 13, 95–100. [Google Scholar] [CrossRef]
- Morris, C.J. Carrageenan-induced paw edema in the rat and mouse. Inflamm. Prot. 2003, 225, 115–121. [Google Scholar]
- Yu, G.; Rao, P.P.; Chowdhury, M.A.; Abdellatif, K.R.; Dong, Y.; Das, D.; Velázquez, C.A.; Suresh, M.R.; Knaus, E.E. Synthesis and biological evaluation of N-difluoromethyl-1, 2-dihydropyrid-2-one acetic acid regioisomers: Dual inhibitors of cyclooxygenases and 5-lipoxygenase. Bioorg. Med. Chem. Lett. 2010, 20, 2168–2173. [Google Scholar] [CrossRef]
- Epifano, F.; Genovese, S.; Sosa, S.; Tubaro, A.; Curini, M. Synthesis and anti-inflammatory activity of 3-(4′-geranyloxy-3′-methoxyphenyl)-2-trans propenoic acid and its ester derivatives. Bioorg. Med. Chem. Lett. 2007, 17, 5709–5714. [Google Scholar] [CrossRef] [PubMed]
- Sala, A.; Recio, M.C.; Schinella, G.R.; Máñez, S.; Giner, R.M.; Ríos, J.-L. A new dual inhibitor of arachidonate metabolism isolated from Helichrysum italicum. Eur. J. Pharmacol. 2003, 460, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Rossi, P.-G.; Berti, L.; Panighi, J.; Luciani, A.; Maury, J.; Muselli, A.; Serra, D.d.R.; Gonny, M.; Bolla, J.-M. Antibacterial action of essential oils from Corsica. J. Essent. Oil Res. 2007, 19, 176–182. [Google Scholar] [CrossRef]
- Chao, S.; Young, G.; Oberg, C.; Nakaoka, K. Inhibition of methicillin-resistant Staphylococcus aureus (MRSA) by essential oils. Flavour Fragr. J. 2008, 23, 444–449. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.; Musolino, A.; Procopio, F.; Alonzo, V. Helichrysum italicum extract interferes with the production of enterotoxins by Staphylococcus aureus. Lett. Appl. Microbiol. 2002, 35, 181–184. [Google Scholar] [CrossRef]
- Nostro, A.; Cannatelli, M.; Crisafi, G.; Musolino, A.; Procopio, F.; Alonzo, V. Modifications of hydrophobicity, in vitro adherence and cellular aggregation of Streptococcus mutans by Helichrysum italicum extract. Lett. Appl. Microbiol. 2004, 38, 423–427. [Google Scholar] [CrossRef]
- Generalić Mekinić, I.; Skroza, D.; Ljubenkov, I.; Šimat, V.; Smole Možina, S.; Katalinić, V. In vitro antioxidant and antibacterial activity of Lamiaceae phenolic extracts: A correlation study. Food Technol. Biotechnol. 2014, 52, 119–127. [Google Scholar]
- Mucsi, I.; Gyulai, Z.; Beladi, I. Combined effects of flavonoids and acyclovir against herpesviruses in cell cultures. Acta Microbiol. Hung. 1992, 39, 137–147. [Google Scholar]
- Molnar, M.; Jerković, I.; Suknović, D.; Bilić Rajs, B.; Aladić, K.; Šubarić, D.; Jokić, S. Screening of Six Medicinal Plant Extracts Obtained by Two Conventional Methods and Supercritical CO2 Extraction Targeted on Coumarin Content, 2, 2-Diphenyl-1-picrylhydrazyl Radical Scavenging Capacity and Total Phenols Content. Molecules 2017, 22, 348. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves, S.; Moreira, E.; Grosso, C.; Andrade, P.B.; Valentão, P.; Romano, A. Phenolic profile, antioxidant activity and enzyme inhibitory activities of extracts from aromatic plants used in Mediterranean diet. J. Food Sci. Technol. 2017, 54, 219–227. [Google Scholar] [CrossRef]
- Lešnik, S.; Furlan, V.; Bren, U. Rosemary (Rosmarinus officinalis L.): Extraction techniques, analytical methods and health-promoting biological effects. Phytochem. Rev. 2021, 20, 1273–1328. [Google Scholar] [CrossRef]
- Sandle, T. European Pharmacopoeia, 9th ed.; Council of Europe: Strasburg, France, 2011. [Google Scholar]
- World Health Organization. WHO Guidelines on Good Agricultural and Collection Practices (GACP) for Medicinal Plants; World Health Organization: Geneva, Switzerland, 2003.
- Ramos, S. Cancer chemoprevention and chemotherapy: Dietary polyphenols and signalling pathways. Mol. Nutr. Food Res. 2008, 52, 507–526. [Google Scholar] [CrossRef] [PubMed]
- Brglez Mojzer, E.; Knez Hrnčič, M.; Škerget, M.; Knez, Ž.; Bren, U. Polyphenols: Extraction methods, antioxidative action, bioavailability and anticarcinogenic effects. Molecules 2016, 21, 901. [Google Scholar] [CrossRef]
- Robbins, R.J. Phenolic acids in foods: An overview of analytical methodology. J. Agric. Food Chem. 2003, 51, 2866–2887. [Google Scholar] [CrossRef]
- Di Modica, G.; Tira, S. Sostanze isolate da Helichrysum italicum G. Don: Frazini neutre. Anal. Chim. 1958, 48, 681–689. [Google Scholar]
- Vanucci-Bacqué, C.; Carayon, C.; Bernis, C.; Camare, C.; Nègre-Salvayre, A.; Bedos-Belval, F.; Baltas, M. Synthesis, antioxidant and cytoprotective evaluation of potential antiatherogenic phenolic hydrazones. A structure–activity relationship insight. Biorg. Med. Chem. 2014, 22, 4269–4276. [Google Scholar] [CrossRef]
- Cardoso, C.L.; Castro-Gamboa, I.; Bergamini, G.M.; Cavalheiro, A.J.; Silva, D.H.; Lopes, M.N.; Araujo, A.R.; Furlan, M.; Verli, H.; Bolzani, V.d.S. An Unprecedented Neolignan Skeleton from Chimarrhis turbinata. J. Nat. Prod. 2011, 74, 487–491. [Google Scholar] [CrossRef]
- Luyen, B.T.T.; Tai, B.H.; Thao, N.P.; Cha, J.Y.; Lee, H.Y.; Lee, Y.M.; Kim, Y.H. Anti-inflammatory components of Chrysanthemum indicum flowers. Bioorg. Med. Chem. Lett. 2015, 25, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Huss, U.; Ringbom, T.; Perera, P.; Bohlin, L.; Vasänge, M. Screening of ubiquitous plant constituents for COX-2 inhibition with a scintillation proximity based assay. J. Nat. Prod. 2002, 65, 1517–1521. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Yu, S.; Liang, Y.; Huang, H.; Lian, X.-Y.; Zhang, Z. Bioactive triterpenoid saponins and phenolic compounds against glioma cells. Bioorg. Med. Chem. Lett. 2014, 24, 5157–5163. [Google Scholar] [CrossRef] [PubMed]
- D’Abrosca, B.; Buommino, E.; D’Angelo, G.; Coretti, L.; Scognamiglio, M.; Severino, V.; Pacifico, S.; Donnarumma, G.; Fiorentino, A. Spectroscopic identification and anti-biofilm properties of polar metabolites from the medicinal plant Helichrysum italicum against Pseudomonas aeruginosa. Biorg. Med. Chem. 2013, 21, 7038–7046. [Google Scholar] [CrossRef]
- Konstantinopoulou, M.; Karioti, A.; Skaltsas, S.; Skaltsa, H. Sesquiterpene Lactones from Anthemis a ltissima and Their Anti-Helicobacter p ylori Activity. J. Nat. Prod. 2003, 66, 699–702. [Google Scholar] [CrossRef]
- Ma, C.-M.; Kully, M.; Khan, J.K.; Hattori, M.; Daneshtalab, M. Synthesis of chlorogenic acid derivatives with promising antifungal activity. Biorg. Med. Chem. 2007, 15, 6830–6833. [Google Scholar] [CrossRef]
- King, P.J.; Ma, G.; Miao, W.; Jia, Q.; McDougall, B.R.; Reinecke, M.G.; Cornell, C.; Kuan, J.; Kim, T.R.; Robinson, W.E. Structure− activity relationships: Analogues of the dicaffeoylquinic and dicaffeoyltartaric acids as potent inhibitors of human immunodeficiency virus type 1 integrase and replication. J. Med. Chem. 1999, 42, 497–509. [Google Scholar] [CrossRef]
- Pereira, C.G.; Barreira, L.; Bijttebier, S.; Pieters, L.; Neves, V.; Rodrigues, M.J.; Rivas, R.; Varela, J.; Custódio, L. Chemical profiling of infusions and decoctions of Helichrysum italicum subsp. picardii by UHPLC-PDA-MS and in vitro biological activities comparatively with green tea (Camellia sinensis) and rooibos tisane (Aspalathus linearis). J. Pharm. Biomed. Anal. 2017, 145, 593–603. [Google Scholar] [CrossRef]
- Georgiev, L.; Chochkova, M.; Totseva, I.; Seizova, K.; Marinova, E.; Ivanova, G.; Ninova, M.; Najdenski, H.; Milkova, T. Anti-tyrosinase, antioxidant and antimicrobial activities of hydroxycinnamoylamides. Med. Chem. Res. 2013, 22, 4173–4182. [Google Scholar] [CrossRef]
- Digiacomo, M.; Chen, Z.; Wang, S.; Lapucci, A.; Macchia, M.; Yang, X.; Chu, J.; Han, Y.; Pi, R.; Rapposelli, S. Synthesis and pharmacological evaluation of multifunctional tacrine derivatives against several disease pathways of AD. Bioorg. Med. Chem. Lett. 2015, 25, 807–810. [Google Scholar] [CrossRef]
- Bora-Tatar, G.; Dayangaç-Erden, D.; Demir, A.S.; Dalkara, S.; Yelekçi, K.; Erdem-Yurter, H. Molecular modifications on carboxylic acid derivatives as potent histone deacetylase inhibitors: Activity and docking studies. Biorg. Med. Chem. 2009, 17, 5219–5228. [Google Scholar] [CrossRef] [PubMed]
- Thao, N.P.; Tai, B.H.; Luyen, B.T.T.; Kim, S.; Koo, J.E.; Koh, Y.S.; Cuong, N.T.; Van Thanh, N.; Cuong, N.X.; Nam, N.H. Chemical constituents from Kandelia candel with their inhibitory effects on pro-inflammatory cytokines production in LPS-stimulated bone marrow-derived dendritic cells (BMDCs). Bioorg. Med. Chem. Lett. 2015, 25, 1412–1416. [Google Scholar]
- Chen, H.; Li, G.; Zhan, P.; Li, H.; Wang, S.; Liu, X. Design, synthesis and biological evaluation of novel trimethylpyrazine-2-carbonyloxy-cinnamic acids as potent cardiovascular agents. Med. Chem. Comm. 2014, 5, 711–718. [Google Scholar] [CrossRef]
- Miyamae, Y.; Kurisu, M.; Murakami, K.; Han, J.; Isoda, H.; Irie, K.; Shigemori, H. Protective effects of caffeoylquinic acids on the aggregation and neurotoxicity of the 42-residue amyloid β-protein. Biorg. Med. Chem. 2012, 20, 5844–5849. [Google Scholar] [CrossRef] [PubMed]
- Miliovsky, M.; Svinyarov, I.; Mitrev, Y.; Evstatieva, Y.; Nikolova, D.; Chochkova, M.; Bogdanov, M.G. A novel one-pot synthesis and preliminary biological activity evaluation of cis-restricted polyhydroxy stilbenes incorporating protocatechuic acid and cinnamic acid fragments. Eur. J. Med. Chem. 2013, 66, 185–192. [Google Scholar] [CrossRef]
- Srivastava, V.; Darokar, M.P.; Fatima, A.; Kumar, J.; Chowdhury, C.; Saxena, H.O.; Dwivedi, G.R.; Shrivastava, K.; Gupta, V.; Chattopadhyay, S. Synthesis of diverse analogues of Oenostacin and their antibacterial activities. Biorg. Med. Chem. 2007, 15, 518–525. [Google Scholar] [CrossRef] [PubMed]
- Fu, J.; Cheng, K.; Zhang, Z.-m.; Fang, R.-q.; Zhu, H.-l. Synthesis, structure and structure–activity relationship analysis of caffeic acid amides as potential antimicrobials. Eur. J. Med. Chem. 2010, 45, 2638–2643. [Google Scholar] [CrossRef] [PubMed]
- Queffélec, C.; Bailly, F.; Mbemba, G.; Mouscadet, J.-F.; Hayes, S.; Debyser, Z.; Witvrouw, M.; Cotelle, P. Synthesis and antiviral properties of some polyphenols related to Salvia genus. Bioorg. Med. Chem. Lett. 2008, 18, 4736–4740. [Google Scholar] [CrossRef]
- Singh, M.; Kaur, M.; Silakari, O. Flavones: An important scaffold for medicinal chemistry. Eur. J. Med. Chem. 2014, 84, 206–239. [Google Scholar] [CrossRef]
- Wollenweber, E.; Christ, M.; Dunstan, R.H.; Roitman, J.N.; Stevens, J.F. Exudate flavonoids in some Gnaphalieae and Inuleae (Asteraceae). Z. Nat. C 2005, 60, 671–678. [Google Scholar] [CrossRef]
- Shin, S.Y.; Woo, Y.; Hyun, J.; Yong, Y.; Koh, D.; Lee, Y.H.; Lim, Y. Relationship between the structures of flavonoids and their NF-κB-dependent transcriptional activities. Bioorg. Med. Chem. Lett. 2011, 21, 6036–6041. [Google Scholar] [CrossRef] [PubMed]
- Cos, P.; Ying, L.; Calomme, M.; Hu, J.P.; Cimanga, K.; Van Poel, B.; Pieters, L.; Vlietinck, A.J.; Berghe, D.V. Structure− activity relationship and classification of flavonoids as inhibitors of xanthine oxidase and superoxide scavengers. J. Nat. Prod. 1998, 61, 71–76. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.X.; Lu, J.C.; Fang, Z.Z.; Zhang, Y.Y.; Cao, Y.F.; Mao, Y.X.; Zhu, L.L.; Yin, J.; Yang, L. Reversible inhibition of three important human liver cytochrome p450 enzymes by tiliroside. Phytother. Res. 2010, 24, 1670–1675. [Google Scholar] [CrossRef] [PubMed]
- Takemura, H.; Itoh, T.; Yamamoto, K.; Sakakibara, H.; Shimoi, K. Selective inhibition of methoxyflavonoids on human CYP1B1 activity. Biorg. Med. Chem. 2010, 18, 6310–6315. [Google Scholar] [CrossRef]
- Chen, W.-Q.; Song, Z.-J.; Xu, H.-H. A new antifungal and cytotoxic C-methylated flavone glycoside from Picea neoveitchii. Bioorg. Med. Chem. Lett. 2012, 22, 5819–5822. [Google Scholar] [CrossRef]
- Ramírez-Galicia, G.; Martínez-Pacheco, H.; Garduño-Juárez, R.; Deeb, O. Exploring QSAR of antiamoebic agents of isolated natural products by MLR, ANN, and RTO. Med. Chem. Res. 2012, 21, 2501–2516. [Google Scholar] [CrossRef]
- Freitas, R.F.; Prokopczyk, I.M.; Zottis, A.; Oliva, G.; Andricopulo, A.D.; Trevisan, M.T.S.; Vilegas, W.; Silva, M.G.V.; Montanari, C.A. Discovery of novel Trypanosoma cruzi glyceraldehyde-3-phosphate dehydrogenase inhibitors. Biorg. Med. Chem. 2009, 17, 2476–2482. [Google Scholar] [CrossRef]
- Tan, G.T.; Pezzuto, J.M.; Kinghorn, A.D.; Hughes, S.H. Evaluation of natural products as inhibitors of human immunodeficiency virus type 1 (HIV-1) reverse transcriptase. J. Nat. Prod. 1991, 54, 143–154. [Google Scholar] [CrossRef]
- Li, B.-W.; Zhang, F.-H.; Serrao, E.; Chen, H.; Sanchez, T.W.; Yang, L.-M.; Neamati, N.; Zheng, Y.-T.; Wang, H.; Long, Y.-Q. Design and discovery of flavonoid-based HIV-1 integrase inhibitors targeting both the active site and the interaction with LEDGF/p75. Bioorg. Med. Chem. 2014, 22, 3146–3158. [Google Scholar] [CrossRef]
- Li, X.-C.; Joshi, A.S.; ElSohly, H.N.; Khan, S.I.; Jacob, M.R.; Zhang, Z.; Khan, I.A.; Ferreira, D.; Walker, L.A.; Broedel, S.E. Fatty acid synthase inhibitors from plants: Isolation, structure elucidation, and SAR studies. J. Nat. Prod. 2002, 65, 1909–1914. [Google Scholar] [CrossRef]
- Werner, J.; Ebrahim, W.; Özkaya, F.C.; Mándi, A.; Kurtán, T.; El-Neketi, M.; Liu, Z.; Proksch, P. Pyrone derivatives from Helichrysum italicum. Fitoterapia 2019, 133, 80–84. [Google Scholar] [CrossRef]
- Liobikas, J.; Majiene, D.; Trumbeckaite, S.; Kursvietiene, L.; Masteikova, R.; Kopustinskiene, D.M.; Savickas, A.; Bernatoniene, J. Uncoupling and antioxidant effects of ursolic acid in isolated rat heart mitochondria. J. Nat. Prod. 2011, 74, 1640–1644. [Google Scholar] [CrossRef] [PubMed]
- Pedada, S.R.; Yarla, N.S.; Tambade, P.J.; Dhananjaya, B.L.; Bishayee, A.; Arunasree, K.M.; Philip, G.H.; Dharmapuri, G.; Aliev, G.; Putta, S. Synthesis of new secretory phospholipase A 2-inhibitory indole containing isoxazole derivatives as anti-inflammatory and anticancer agents. Eur. J. Med. Chem. 2016, 112, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.; Chattopadhyay, D.; Mandal, A.; Kaity, S.; Samanta, A. Bioactivity guided isolation of antiinflammatory, analgesic, and antipyretic constituents from the leaves of Pedilanthus tithymaloides (L.). Med. Chem. Res. 2013, 22, 4347–4359. [Google Scholar] [CrossRef]
- Acebey-Castellon, I.L.; Voutquenne-Nazabadioko, L.; Doan Thi Mai, H.; Roseau, N.; Bouthagane, N.; Muhammad, D.; Le Magrex Debar, E.; Gangloff, S.C.; Litaudon, M.; Sevenet, T. Triterpenoid saponins from Symplocos lancifolia. J. Nat. Prod. 2011, 74, 163–168. [Google Scholar] [CrossRef] [PubMed]
- Chien, N.Q.; Hung, N.V.; Santarsiero, B.D.; Mesecar, A.D.; Cuong, N.M.; Soejarto, D.D.; Pezzuto, J.M.; Fong, H.H.; Tan, G.T. New 3-O-acyl betulinic acids from Strychnos vanprukii Craib. J. Nat. Prod. 2004, 67, 994–998. [Google Scholar] [CrossRef]
- de Brum Vieira, P.; Silva, N.L.F.; da Silva, G.N.S.; Silva, D.B.; Lopes, N.P.; Gnoatto, S.C.B.; da Silva, M.V.; Macedo, A.J.; Bastida, J.; Tasca, T. Caatinga plants: Natural and semi-synthetic compounds potentially active against Trichomonas vaginalis. Bioorg. Med. Chem. Lett. 2016, 26, 2229–2236. [Google Scholar] [CrossRef]
- Kwon, S.-H.; Park, H.-Y.; Kim, J.-Y.; Jeong, I.-Y.; Lee, M.-K.; Seo, K.-I. Apoptotic action of ursolic acid isolated from Corni fructus in RC-58T/h/SA# 4 primary human prostate cancer cells. Bioorg. Med. Chem. Lett. 2010, 20, 6435–6438. [Google Scholar]
- Bai, K.-K.; Yu, Z.; Chen, F.-L.; Li, F.; Li, W.-Y.; Guo, Y.-H. Synthesis and evaluation of ursolic acid derivatives as potent cytotoxic agents. Bioorg. Med. Chem. Lett. 2012, 22, 2488–2493. [Google Scholar] [CrossRef]
- Yang, H.; Jeong, E.J.; Kim, J.; Sung, S.H.; Kim, Y.C. Antiproliferative triterpenes from the leaves and twigs of Juglans sinensis on HSC-T6 cells. J. Nat. Prod. 2011, 74, 751–756. [Google Scholar] [CrossRef]
- Wiemann, J.; Heller, L.; Csuk, R. Targeting cancer cells with oleanolic and ursolic acid derived hydroxamates. Bioorg. Med. Chem. Lett. 2016, 26, 907–909. [Google Scholar] [CrossRef]
- Estevinho, L.; Pereira, A.P.; Moreira, L.; Dias, L.G.; Pereira, E. Antioxidant and antimicrobial effects of phenolic compounds extracts of Northeast Portugal honey. Food Chem. Toxicol. 2008, 46, 3774–3779. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.-L.; Qiang, G.-F.; Gao, M.; Zhang, H.-A.; Chen, B.-N.; Yu, X.-Y.; Xuan, Z.-H.; Wang, Q.-Y.; Du, G.-H. Effect of pinocembrin on brain mitochondrial respiratory function. Yao Xue Xue Bao = Acta Pharm. Sin. 2011, 46, 642–649. [Google Scholar]
- Heller, L.; Schwarz, S.; Perl, V.; Köwitsch, A.; Siewert, B.; Csuk, R. Incorporation of a Michael acceptor enhances the antitumor activity of triterpenoic acids. Eur. J. Med. Chem. 2015, 101, 391–399. [Google Scholar] [CrossRef] [PubMed]
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils–a review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Primitivo, M.J.; Neves, M.; Pires, C.L.; Cruz, P.F.; Brito, C.; Rodrigues, A.C.; de Carvalho, C.C.; Mortimer, M.M.; Moreno, M.J.; Brito, R.M. Edible flowers of Helichrysum italicum: Composition, nutritive value, and bioactivities. Food Res. Int. 2022, 157, 111399. [Google Scholar] [CrossRef]
- Fraternale, D.; Flamini, G.; Ascrizzi, R. In Vitro anticollagenase and antielastase activities of essential oil of Helichrysum italicum subsp. italicum (Roth) G. Don. J. Med. Food 2019, 22, 1041–1046. [Google Scholar] [CrossRef]
- Andreani, S.; Uehara, A.; Blagojević, P.; Radulović, N.; Muselli, A.; Baldovini, N. Key odorants of industrially-produced Helichrysum italicum subsp. italicum essential oil. Ind. Crops Prod. 2019, 132, 275–282. [Google Scholar] [CrossRef]
- Masotti, V.; Juteau, F.; Bessière, J.M.; Viano, J. Seasonal and phenological variations of the essential oil from the narrow endemic species Artemisia molinieri and its biological activities. J. Agric. Food Chem. 2003, 51, 7115–7121. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Coroneo, V.; Dessi, S.; Cabras, P. Chemical composition, seasonal variability, and antifungal activity of Lavandula stoechas L. ssp. stoechas essential oils from stem/leaves and flowers. J. Agric. Food Chem. 2006, 54, 4364–4370. [Google Scholar] [CrossRef]
- Rhind, J.P. Essential Oils: A Handbook for Aromatherapy Practice, 2nd ed.; Singing Dragon: London, UK, 2012. [Google Scholar]
- Han, X.; Rodriguez, D.; Parker, T.L. Biological activities of frankincense essential oil in human dermal fibroblasts. Biochim. Open 2017, 4, 31–35. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Pavela, R.; Giordani, C.; Casettari, L.; Curzi, G.; Cappellacci, L.; Petrelli, R.; Maggi, F. Acute and sub-lethal toxicity of eight essential oils of commercial interest against the filariasis mosquito Culex quinquefasciatus and the housefly Musca domestica. Ind. Crops Prod. 2018, 112, 668–680. [Google Scholar] [CrossRef]
- Giraud-Robert, A. The role of aromatherapy in the treatment of viral hepatitis. Int. J. Aromather. 2005, 15, 183–192. [Google Scholar] [CrossRef]
- Idaomar, M.; El Hamss, R.; Bakkali, F.; Mezzoug, N.; Zhiri, A.; Baudoux, D.; Munoz-Serrano, A.; Liemans, V.; Alonso-Moraga, A. Genotoxicity and antigenotoxicity of some essential oils evaluated by wing spot test of Drosophila melanogaster. Mutat. Res. Genet. Toxicol. Environ. Mutagen. 2002, 513, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Lajovic, A.; Nagy, L.D.; Guengerich, F.P.; Bren, U. Carcinogenesis of urethane: Simulation versus experiment. Chem. Res. Toxicol. 2015, 28, 691. [Google Scholar] [CrossRef] [PubMed]
- Foti, C.; Guida, S.; Antelmi, A.; Romita, P.; Corazza, M. Allergic contact dermatitis caused by Helichrysum italicum contained in an emollient cream. Contact Derm. 2013, 69, 62–63. [Google Scholar] [CrossRef]
- Ipek, E.; Zeytinoglu, H.; Okay, S.; Tuylu, B.A.; Kurkcuoglu, M.; Baser, K.H.C. Genotoxicity and antigenotoxicity of Origanum oil and carvacrol evaluated by Ames Salmonella/microsomal test. Food Chem. 2005, 93, 551–556. [Google Scholar] [CrossRef]
- Cal, K. Skin penetration of terpenes from essential oils and topical vehicles. Planta Med. 2006, 72, 311–316. [Google Scholar] [CrossRef]
- Mancini, E.; De Martino, L.; Marandino, A.; Scognamiglio, M.R.; De Feo, V. Chemical composition and possible in vitro phytotoxic activity of Helichrsyum italicum (Roth) Don ssp. italicum. Molecules 2011, 16, 7725–7735. [Google Scholar] [CrossRef] [Green Version]
- Bianchini, A.; Tomi, P.; Bernardini, A.F.; Morelli, I.; Flamini, G.; Cioni, P.L.; Usaï, M.; Marchetti, M. A comparative study of volatile constituents of two Helichrysum italicum (Roth) Guss. Don Fil subspecies growing in Corsica (France), Tuscany and Sardinia (Italy). Flavour Fragr. J. 2003, 18, 487–491. [Google Scholar] [CrossRef]
- Bianchini, A.; Tomi, P.; Costa, J.; Bernardini, A.F. Composition of Helichrysum italicum (Roth) G. Don fil. subsp. italicum essential oils from Corsica (France). Flavour Fragr. J. 2001, 16, 30–34. [Google Scholar] [CrossRef]
- Chinou, I.B.; Roussis, V.; Perdetzoglou, D.; Loukis, A. Chemical and biological studies on two Helichrysum species of Greek origin. Planta Med. 1996, 62, 377–379. [Google Scholar] [CrossRef] [PubMed]
- Usai, M.; Foddai, M.; Bernardini, A.; Muselli, A.; Costa, J.; Marchetti, M. Chemical composition and variation of the essential oil of wild sardinian Helichrysum italicum G. Don subsp. microphyllum (Willd.) Nym from vegetative period to post-blooming. J. Essent. Oil Res. 2010, 22, 373–380. [Google Scholar] [CrossRef]
- Satta, M.; Tuberoso, C.; Angioni, A.; Pirisi, F.; Cabras, P. Analysis of the Essential Oil of Helichrysum italicum G. Don ssp. microphyllum (Willd) Nym. J. Essent. Oil Res. 1999, 11, 711–715. [Google Scholar] [CrossRef]
- Blazevic, N.; Petricic, J.; Stanic, G.; Males, Z. Variations in yields and composition of immortelle (Helichrysum italicum, Roth Guss.) essential oil from different locations and vegetation periods along Adriatic coast. Acta Pharm. 1995, 45, 517–522. [Google Scholar]
- Hladnik, M.; Baruca Arbeiter, A.; Knap, T.; Jakše, J.; Bandelj, D. The complete chloroplast genome of Helichrysum italicum (Roth) G. Don (Asteraceae). Mitochondrial DNA B 2019, 4, 1036–1037. [Google Scholar] [CrossRef]
- Kordali, S.; Kesdek, M.; Cakir, A. Toxicity of monoterpenes against larvae and adults of Colorado potato beetle, Leptinotarsa decemlineata Say (Coleoptera: Chrysomelidae). Ind. Crops Prod. 2007, 26, 278–297. [Google Scholar] [CrossRef]
- Ramos Alvarenga, R.F.; Wan, B.; Inui, T.; Franzblau, S.G.; Pauli, G.F.; Jaki, B.U. Airborne antituberculosis activity of Eucalyptus citriodora essential oil. J. Nat. Prod. 2014, 77, 603–610. [Google Scholar] [CrossRef]
- Perrucci, S.; Macchioni, G.; Cioni, P.L.; Flamini, G.; Morelli, I. Structure/activity relationship of some natural monoterpenes as acaricides against Psoroptes cuniculi. J. Nat. Prod. 1995, 58, 1261–1264. [Google Scholar] [CrossRef]
- Caballero-Gallardo, K.; Olivero-Verbel, J.; Stashenko, E.E. Repellent activity of essential oils and some of their individual constituents against Tribolium castaneum Herbst. J. Agric. Food Chem. 2011, 59, 1690–1696. [Google Scholar] [CrossRef]
- Ortar, G.; Moriello, A.S.; Morera, E.; Nalli, M.; Di Marzo, V.; De Petrocellis, L. Effect of acyclic monoterpene alcohols and their derivatives on TRP channels. Bioorg. Med. Chem. Lett. 2014, 24, 5507–5511. [Google Scholar] [CrossRef] [PubMed]
- Bauer, K.G.; Garbe, D.; Surburg, H. Common Fragrance and Flavor Materials: Preparation, Properties and Uses; Wiley-VCH: Weinheim, Germany, 2001. [Google Scholar]
- Baser, K.H.C.; Buchbauer, G. Handbook of Essential Oils: Science, Technology, and Applications; CRC Press: Boca Raton, FL, USA, 2015. [Google Scholar]
- Burits, M.; Asres, K.; Bucar, F. The antioxidant activity of the essential oils of Artemisia afra, Artemisia abyssinica and Juniperus procera. Phytother. Res. 2001, 15, 103–108. [Google Scholar] [CrossRef] [PubMed]
- De-Oliveira, A.C.; Ribeiro-Pinto, L.F.; Paumgartten, F.J. In vitro inhibition of CYP2B1 monooxygenase by β-myrcene and other monoterpenoid compounds. Toxicol. Lett. 1997, 92, 39–46. [Google Scholar] [CrossRef] [PubMed]
- Lorente, I.; Ocete, M.; Zarzuelo, A.; Cabo, M.; Jimenez, J. Bioactivity of the essential oil of Bupleurum fruticosum. J. Nat. Prod. 1989, 52, 267–272. [Google Scholar] [CrossRef]
- Rufino, A.T.; Ribeiro, M.; Judas, F.; Salgueiro, L.; Lopes, M.C.; Cavaleiro, C.; Mendes, A.F. Anti-inflammatory and chondroprotective activity of (+)-α-pinene: Structural and enantiomeric selectivity. J. Nat. Prod. 2014, 77, 264–269. [Google Scholar] [CrossRef]
- Angioni, A.; Barra, A.; Cereti, E.; Barile, D.; Coïsson, J.D.; Arlorio, M.; Dessi, S.; Coroneo, V.; Cabras, P. Chemical composition, plant genetic differences, antimicrobial and antifungal activity investigation of the essential oil of Rosmarinus officinalis L. J. Agric. Food Chem. 2004, 52, 3530–3535. [Google Scholar] [CrossRef]
- Schnuch, A.; Uter, W.; Geier, J.; Lessmann, H.; Frosch, P.J. Sensitization to 26 fragrances to be labelled according to current European regulation. Contact Derm. 2007, 57, 1–10. [Google Scholar] [CrossRef]
- Matura, M.; Sköld, M.; Börje, A.; Andersen, K.E.; Bruze, M.; Frosch, P.; Goossens, A.; Johansen, J.D.; Svedman, C.; White, I.R. Not only oxidized R-(+)-but also S-(−)-limonene is a common cause of contact allergy in dermatitis patients in Europe. Contact Derm. 2006, 55, 274–279. [Google Scholar] [CrossRef]
- Souza, M.; Siani, A.C.; Ramos, M.; Menezes-de-Lima Jr, O.; Henriques, M. Evaluation of anti-inflammatory activity of essential oils from two Asteraceae species. Int. J. Pharm. Sci. 2003, 58, 582–586. [Google Scholar]
- Wilkins, J.S., Jr. Method for Treating Gastrointestinal Disorders. 2002. Available online: https://patents.google.com/patent/US6420435B1/en (accessed on 9 February 2023).
- Joint, F. Summary and Conclusions of the Sixty-First Meeting of the Joint FAO/WHO Expert Committee on Food Additives (JECFA); JECFA: Rome, Italy, 2003. [Google Scholar]
- Joint FAO/WHO Expert Committee on Food Additives, World Health Organization & Food and Agriculture Organization of the United Nations. Evaluation of Certain Food Additives and Contaminants: Forty-Sixth Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 1997.
- WHO, FAO, Joint FAO/WHO Expert Committee on Food Additives, Joint FAO/WHO Expert Committee on Food Additives. Evaluation of Certain Food Additives and Contaminants: Sixty-First Report of the Joint FAO/WHO Expert Committee on Food Additives; World Health Organization: Geneva, Switzerland, 2004; Volume 61.
- Ohara, M.; Ohyama, Y. Delivery and application of dietary polyphenols to target organs, tissues and intracellular organelles. Curr. Drug Metab. 2014, 15, 37–47. [Google Scholar] [CrossRef]
- Saraf, S. Applications of novel drug delivery system for herbal formulations. Fitoterapia 2010, 81, 680–689. [Google Scholar]
- Kupnik, K.; Primožič, M.; Kokol, V.; Leitgeb, M. Nanocellulose in drug delivery and antimicrobially active materials. Polymers 2020, 12, 2825. [Google Scholar] [CrossRef] [PubMed]
- Karača, S.; Bušić, A.; Đorđević, V.; Belščak-Cvitanović, A.; Cebin, A.V.; Bugarski, B.; Komes, D. The functional potential of immortelle (Helichrysum italicum) based edible films reinforced with proteins and hydrogel particles. LWT 2019, 99, 387–395. [Google Scholar] [CrossRef]
- Maleki, G.; Woltering, E.J.; Mozafari, M. Applications of chitosan-based carrier as an encapsulating agent in food industry. Trends Food Sci. Technol. 2022, 120, 88–99. [Google Scholar] [CrossRef]
- Di Santo, M.C.; D’Antoni, C.L.; Rubio, A.P.D.; Alaimo, A.; Pérez, O.E. Chitosan-tripolyphosphate nanoparticles designed to encapsulate polyphenolic compounds for biomedical and pharmaceutical applications− A review. Biomed. Pharmacother. 2021, 142, 111970. [Google Scholar] [CrossRef]
- Hosseini, S.; Varidi, M. Optimization of microbial rennet encapsulation in alginate–chitosan nanoparticles. Food Chem. 2021, 352, 129325. [Google Scholar] [CrossRef]
- Carrasco-Sandoval, J.; Aranda-Bustos, M.; Henríquez-Aedo, K.; López-Rubio, A.; Fabra, M.J. Bioaccessibility of different types of phenolic compounds co-encapsulated in alginate/chitosan-coated zein nanoparticles. LWT 2021, 149, 112024. [Google Scholar] [CrossRef]
- Belščak-Cvitanović, A.; Komes, D.; Karlović, S.; Djaković, S.; Špoljarić, I.; Mršić, G.; Ježek, D. Improving the controlled delivery formulations of caffeine in alginate hydrogel beads combined with pectin, carrageenan, chitosan and psyllium. Food Chem. 2015, 167, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Maja, L.; Željko, K.; Mateja, P. Sustainable technologies for liposome preparation. J. Supercrit. Fluids 2020, 165, 104984. [Google Scholar] [CrossRef]
- Bonechi, C.; Donati, A.; Tamasi, G.; Leone, G.; Consumi, M.; Rossi, C.; Lamponi, S.; Magnani, A. Protective effect of quercetin and rutin encapsulated liposomes on induced oxidative stress. Biophys. Chem. 2018, 233, 55–63. [Google Scholar] [CrossRef]
- Păvăloiu, R.-D.; Sha’at, F.; Neagu, G.; Deaconu, M.; Bubueanu, C.; Albulescu, A.; Sha’at, M.; Hlevca, C. Encapsulation of polyphenols from Lycium barbarum leaves into liposomes as a strategy to improve their delivery. Nanomaterials 2021, 11, 1938. [Google Scholar] [CrossRef] [PubMed]
- Jahanfar, S.; Gahavami, M.; Khosravi-Darani, K.; Jahadi, M.; Mozafari, M. Entrapment of rosemary extract by liposomes formulated by Mozafari method: Physicochemical characterization and optimization. Heliyon 2021, 7, e08632. [Google Scholar] [CrossRef] [PubMed]
- Faraji, Z.; Shakarami, J.; Varshosaz, J.; Jafari, S. Encapsulation of essential oils of Mentha pulegium and Ferula gummosa using nanoliposome technology as a safe botanical pesticide. J. Appl. Biotechnol. Rep. 2020, 7, 237–242. [Google Scholar]
- Rahimpour, Y.; Hamishehkar, H. Liposomes in cosmeceutics. Expert Opin. Drug Deliv. 2012, 9, 443–455. [Google Scholar] [CrossRef]
- Matouskova, P.; Marova, I.; Bokrova, J.; Benesova, P. Effect of encapsulation on antimicrobial activity of herbal extracts with lysozyme. Food Technol. Biotechnol. 2016, 54, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Furlan, V.; Bren, U. Protective Effects of [6]-Gingerol Against Chemical Carcinogens: Mechanistic Insights. Int. J. Mol. Sci. 2020, 21, 695. [Google Scholar] [CrossRef]
- Štern, A.; Furlan, V.; Novak, M.; Štampar, M.; Kolenc, Z.; Kores, K.; Filipič, M.; Bren, U.; Žegura, B. Chemoprotective Effects of Xanthohumol against the Carcinogenic Mycotoxin Aflatoxin B1. Foods 2021, 10, 1331. [Google Scholar] [CrossRef] [PubMed]
- Furlan, V.; Konc, J.; Bren, U. Inverse molecular docking as a novel approach to study anticarcinogenic and anti-neuroinflammatory effects of curcumin. Molecules 2018, 23, 3351. [Google Scholar] [CrossRef]
- Kores, K.; Kolenc, Z.; Furlan, V.; Bren, U. Inverse Molecular Docking Elucidating the Anticarcinogenic Potential of the Hop Natural Product Xanthohumol and Its Metabolites. Foods 2022, 11, 1253. [Google Scholar] [CrossRef]
- Furlan, V.; Bren, U. Insight into Inhibitory Mechanism of PDE4D by Dietary Polyphenols Using Molecular Dynamics Simulations and Free Energy Calculations. Biomolecules 2021, 11, 479. [Google Scholar] [CrossRef]
- Pantiora, P.; Furlan, V.; Matiadis, D.; Mavroidi, B.; Perperopoulou, F.; Papageorgiou, A.C.; Sagnou, M.; Bren, U.; Pelecanou, M.; Labrou, N.E. Monocarbonyl Curcumin Analogues as Potent Inhibitors against Human Glutathione Transferase P1-1. Antioxidants 2023, 12, 63. [Google Scholar] [CrossRef] [PubMed]





| Major Compounds | Extraction Temperature (°C) | Extraction Pressure (bar) | Extraction Time (h) | Yield (%) | Identification Method | References |
|---|---|---|---|---|---|---|
| Monoterpenes α-Pinene, nerol, neryl acetate, and neryl propanoate Sesquiterpenes α–Selinene, β-selinene, γ-curcumene, nerolidol, acetate *, widdrol *, β-eudesmol* eudesm-5-en-11-ol *, waxes * | 40–60 | 100–200 | 1.5 | 1.37–4.1 | GC-FID, GC-MS | [60] |
| 40 | 150 | 1.7 | 5.7 | GC-FID, GC-MS | [62] | |
| 50 | 90 | 2–4 | 0.4–1 | GC-MS | [63] | |
| 40 | 80–350 | 3 | 0.35 | GC, GC-MS | [64] | |
| 40 | 90–120 | - | 0.36–0.60 | GC-FID, GC-MS | [65] | |
| 40 | 350 | 5.5 | 3.60 ± 0.23 7.14 ± 0.58 * | GC-FID, GC-MS | [66] | |
| Polyphenolic compounds Pyrogallol, chlorogenic acid derivatives, naringenin, pinocembrin, arzanol *, gentisic acid *, caffeic acid *, luteolin *, tiliroside *, quercetin *, kaempferol *, and apigenin * | 40 | 350 | 5.5 | 3.60 ± 0.23 7.14 ± 0.58 * | HPLC-MS | [66] |
| Scopoletin | 35.86–64.14 | 79.3–220.7 | 1.5 | 0.43–6.31 | HPLC, UV-VIS | [67] |
| Compound | Helichrysum italicum Subspecies | Extraction Yield from Starting Plant Material | Known Biological Effects |
|---|---|---|---|
| Phenolic acids | |||
| Caffeic acid | Picardii Subspecies not specified | up to 0.77% [113] up to 0.0067% [49] | Antioxidant activity [114], anti-inflammatory activity [86,117], histone deacetylase inhibition [116], anticancer activity [118], neuroprotective activity [119], antiviral (anti-HIV) activity [123], antibacterial activity [120,121], antifungal activity [122] |
| Chlorogenic acid | Picardii Subspecies not specified | up to 0.015% [113] up to 0.104% [49] | Antioxidant activity [104], anti-inflammatory activity (inhibition of COX2) [107], anticarcinogenic properties (inhibition of cell proliferation) [108], antibacterial activity [109,110], antifungal activity [111] |
| Flavonoids | |||
| Gnaphaliin | Subspecies not specified | up to 0.03% [54] | Antioxidant activity [21], anti-inflammatory activity [21] |
| Tiliroside | Subspecies not specified | up to 0.0063% [54] | Antioxidant activity [21,115], anti-inflammatory activity [21], inhibition of CYP enzymes [128], antifungal activity [130], antiparasitic activity [131,132], antiviral (anti-HIV) activity [133] |
| Naringenin | Subspecies not specified | up to 0.023% [49] | Antioxidant activity [115], anti-inflammatory activity [87,126], inhibition of CYP enzymes [129], antibacterial activity, antifungal activity [135], antiviral (anti-HIV) activity [134] |
| Pinocembrin | Subspecies not specified | Not specified [125] | Antioxidant activity [21], anti-inflammatory activity [21] antibacterial activity [147], neuroprotective activity [148] |
| Acetophenones | |||
| 4-Hydroxy-3-(3-methyl-2-butenyl) acetophenone | Subspecies not specified | 3.64% [53] | Anti-intiiflammaroty activity [88], inhibition of cyclooxygenase-1 (COX1) [88] |
| 4-Hydroxy-3-(2-hydroxy-3-isopentenyl)acetophenone | Subspecies not specified | 0.04% [53] | Anti-inflammatory activity [53] |
| Tremetones | |||
| 12-Hydroxytremetone | Subspecies not specified | 0.18% [53] | Anti-inflammatory activity [88] |
| Pyrones | |||
| Arzanol | Microphyllum | up to 0.32% [13] | Antioxidant activity [71], anti-inflammatory activity (potential inhibitor of pro-inflammatory mediators [10] and inflammatory enzymes COX1, COX2, and 5-LOX) [11], cytotoxic activity against cancer cells [70], antibacterial activity [13], antiviral (anti-HIV) activity [10] |
| Triterpenes | |||
| Ursolic acid | Microphyllum | up to 0.40% [13] | Antioxidant activity [137], anti-inflammatory activity [138], anticancer activity, induction of apoptosis [143], cell cycle arrest [144], antiproliferative activity [145], cytotoxicity to cancer cells [142,146,149], antibacterial activity [140], antiparasitic activity [132,142], antiviral (anti-HIV) activity [141] |
| Compound | Helichrysum italicum Subspecies | Compounds Content in Essential Oil | Know Biological Effects |
|---|---|---|---|
| Monoterpenes | |||
| Nerol | Microphyllum Italicum | up to 14.4% [169] up to 18.8% [4] | Insecticidal activity [173], antimicrobial activity [174], acaricidal activity [175], repellent activity [176], food additive [189] |
| Neryl acetate | Microphyllum Italicum | up to 55.7% [6] up to 45.9% [33] | Insecticidal activity [173], repellent activity [176], the agonist of TRPA1 [177], food additive [190] |
| Neryl propionate | Microphyllum Italicum | up to 11.4% [170] up to 16.4% [44] | Food additive [190] |
| α-Pinene | Italicum | up to 53.5% [166] | Antioxidative activity [180], anti-inflammatory activity [182,183], inhibition of CYP enzymes [181], antimicrobial activity [184], food additive [191] |
| Limonene | Italicum Microphyllum | 12.9% [33] up to 7% [169] | Anti-inflammatory activity [187], gastroprotective effects [188], inhibition of CYP enzymes [181], food additive [191] |
| Sesquiterpenes | |||
| α-Selinene | Microphyllum Italicum | up to 5.4% [45] up to 26.5% [4] | Pheromone [179] |
| β-Selinene | Microphyllum Italicum | up to 17.2% [45] up to 38% [4] | Pheromone [179] |
| γ-Curcumene | Microphyllum Italicum | up to 18.2% [170] up to 41% [4] | Unknown |
| Eudesm-5-en-11-ol | Italicum Microphyllum | up to 17.2% [33] up to 23.5% [169] | Unknown |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Furlan, V.; Bren, U. Helichrysum italicum: From Extraction, Distillation, and Encapsulation Techniques to Beneficial Health Effects. Foods 2023, 12, 802. https://doi.org/10.3390/foods12040802
Furlan V, Bren U. Helichrysum italicum: From Extraction, Distillation, and Encapsulation Techniques to Beneficial Health Effects. Foods. 2023; 12(4):802. https://doi.org/10.3390/foods12040802
Chicago/Turabian StyleFurlan, Veronika, and Urban Bren. 2023. "Helichrysum italicum: From Extraction, Distillation, and Encapsulation Techniques to Beneficial Health Effects" Foods 12, no. 4: 802. https://doi.org/10.3390/foods12040802