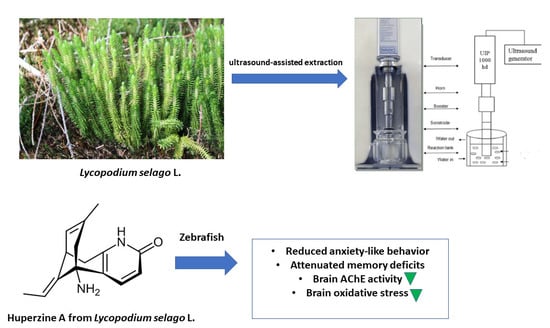
Effects of the Hydroethanolic Extract of Lycopodium selago L. on Scopolamine-Induced Memory Deficits in Zebrafish
Abstract
:1. Introduction
2. Results
2.1. Qualitative Analysis of the Phytochemicals
2.2. Assays for Estimating Antioxidant Activity Using DPPH, FRAP, ABTS, and ORAC
2.3. AChE and BChE Inhibitory Activity
2.4. Total Phenolic and Flavonoid Content
2.5. HPLC/DAD-UV Analysis
2.6. Spatial Memory in Y-Maze, Impact on Anxiety-Like Behavior in NTT and NOR Tests
2.7. Biochemical Parameters Assay in the Brain Tissue
2.8. Pearson Correlations Between Behavioral and Biochemical Parameters
3. Discussion
4. Materials and Methods
4.1. Chemicals and Reagents
4.2. Collection of Plant Material
4.3. Preparation of the Ultrasound-Assisted Hydroethanolic Extract of Lycopodium Selago
4.4. Preliminary Phytochemical Screening
4.5. Measurement of DPPH Radical Scavenging Capacity
4.6. Ferric Reducing Antioxidant Power Assay (FRAP)
4.7. ABTS Radical Scavenging Assay
4.8. The Oxygen Radical Absorbance Capacity (ORAC) Assay
4.9. Investigation of the Inhibitory Actions of Acetylcholinesterase (AChE) and Butyrylcholinesterase (BChE)
4.10. Total Phenolic and Flavonoid Content
4.11. HPLC/DAD-UV Analysis
4.12. Animal Testing and Pharmacological Treatments
4.13. In Vivo Evaluation of the Cognitive Performance
4.14. Y-Maze Test
4.15. Novel Tank Diving Test (NTT)
4.16. Novel Object Recognition Test (NOR)
4.17. Biochemical Parameter Assay
4.18. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Serrano-Pozo, A.; Frosch, M.P.; Masliah, E.; Hyman, B.T. Neuropathological Alterations in Alzheimer Disease. Cold Spring Harb. Perspect. Med. 2011, 1, a006189. [Google Scholar] [CrossRef]
- Ionita, R.; Valu, V.M.; Postu, P.A.; Cioanca, O.; Hritcu, L.; Mihasan, M. 6-Hydroxy-l-nicotine effects on anxiety and depression in a rat model of chlorisondamine. Farmacia 2017, 65, 237–240. [Google Scholar]
- Hussain, R.; Zubair, H.; Pursell, S.; Shahab, M. Neurodegenerative Diseases: Regenerative Mechanisms and Novel Therapeutic Approaches. Brain Sci. 2018, 8, 177. [Google Scholar] [CrossRef] [Green Version]
- Lynch, G. Pharmacological enhancement of memory or cognition in normal subjects. Front. Syst. Neurosci. 2014, 8. [Google Scholar] [CrossRef] [Green Version]
- Soodi, M.; Naghdi, N.; Hajimehdipoor, H.; Choopani, S.; Sahraei, E. Memory-improving activity of Melissa officinalis extract in naïve and scopolamine-treated Rats. Res. Pharm. Sci. 2014, 9, 107–114. [Google Scholar] [PubMed]
- Ferreira-Vieira, T.H.; Guimaraes, I.M.; Silva, F.R.; Ribeiro, F.M. Alzheimer’s disease: Targeting the Cholinergic System. Curr. Neuropharmacol. 2016, 14, 101–115. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hampel, H.; Mesulam, M.-M.; Cuello, A.C.; Farlow, M.R.; Giacobini, E.; Grossberg, G.T.; Khachaturian, A.S.; Vergallo, A.; Cavedo, E.; Snyder, P.J.; et al. The cholinergic system in the pathophysiology and treatment of Alzheimer’s disease. Brain 2018, 141. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Adem, A.; Sabbagh, M. New Acetylcholinesterase Inhibitors for Alzheimer’s Disease. Int. J. Alzheimer’s Dis. 2012, 2012, 728983. [Google Scholar] [CrossRef] [PubMed]
- Colovic, M.B.; Krstic, D.Z.; Lazarevic-Pasti, T.D.; Bondzic, A.M.; Vasic, V.M. Acetylcholinesterase Inhibitors: Pharmacology and Toxicology. Curr. Neuropharmacol. 2013, 11. [Google Scholar] [CrossRef] [Green Version]
- Lleo, A. Current Therapeutic Options for Alzheimers Disease. Curr. Genom. 2007, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Tan, C.; Zhu, D.; Gang, D.R.; Xiao, P. Huperzine A from Huperzia species—An ethnopharmacolgical review. J. Ethnopharmacol. 2007, 113. [Google Scholar] [CrossRef]
- Raves, M.L.; Harel, M.; Pang, Y.P.; Silman, I.; Kozikowski, A.P.; Sussman, J.L. Structure of acetylcholinesterase complexed with the nootropic alkaloid, (-)-huperzine A. Nat. Struct. Biol. 1997, 4, 57–63. [Google Scholar] [CrossRef]
- Ma, X.; Gang, D.R. The Lycopodium alkaloids. Nat. Prod. Rep. 2004, 21, 752–772. [Google Scholar] [CrossRef] [PubMed]
- Ayer, W.A. The Lycopodium alkaloids. Nat. Prod. Rep. 1991, 8, 455–663. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.F.; Qu, S.J.; Xiao, K.; Jiang, S.H.; Tan, J.J.; Tan, C.H.; Zhu, D.Y. Lycopodium alkaloids from Huperzia serrata. J. Asian Nat. Prod. Res. 2010, 12, 1005–1009. [Google Scholar] [CrossRef]
- Soare, L.C.; Ferdes, M.; Stefanov, S.; Denkova, Z.; Nicolova, R.; Denev, P.; Bejan, C.; Paunescu, A. Antioxidant activity, polyphenols content and antimicrobial activity of several native pteridophytes of Romania. Not. Bot. Horti Agrobot. 2012, 40, 53–57. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Wu, H.M.; Zhou, R.L.; Liu, G.J.; Dong, B.R. Huperzine A for Alzheimer’s disease. Cochrane Database Syst. Rev 2008, CD005592. [Google Scholar] [CrossRef]
- Fernandes, R.P.P.; Trindade, M.A.; Tonin, F.G.; Lima, C.G.; Pugine, S.M.P.; Munekata, P.E.S.; Lorenzo, J.M.; de Melo, M.P. Evaluation of antioxidant capacity of 13 plant extracts by three different methods: Cluster analyses applied for selection of the natural extracts with higher antioxidant capacity to replace synthetic antioxidant in lamb burgers. J. Food Sci. Technol. 2016, 53, 451–460. [Google Scholar] [CrossRef] [Green Version]
- Medina-Torres, N.; Ayora-Talavera, T.; Espinosa-Andrews, H.; Sánchez-Contreras, A.; Pacheco, N. Ultrasound Assisted Extraction for the Recovery of Phenolic Compounds from Vegetable Sources. Agronomy 2017, 7, 47. [Google Scholar] [CrossRef]
- Li, H.Z.; Zhang, Z.J.; Xue, J.; Cui, L.X.; Hou, T.Y.; Li, X.J.; Chen, T. Optimization of ultrasound-assisted extraction of phenolic compounds, antioxidants and rosmarinic acid from perilla leaves using response surface methodology. Food Sci. Technol. 2016, 36, 686–693. [Google Scholar] [CrossRef] [Green Version]
- Xu, M.; Eiriksson, F.F.; Thorsteinsdottir, M.; Heidmarsson, S.; Omarsdottir, S.; Olafsdottir, E.S. Alkaloid fingerprinting resolves Huperzia selago genotypes in Iceland. Biochem. Syst. Ecol. 2019, 83, 77–82. [Google Scholar] [CrossRef]
- Valu, M.-V.; Soare, L.C.; Sutan, N.A.; Ducu, C.; Moga, S.; Hritcu, L.; Boiangiu, R.S.; Carradori, S. Optimization of Ultrasonic Extraction to Obtain Erinacine A and Polyphenols with Antioxidant Activity from the Fungal Biomass of Hericium erinaceus. Foods 2020, 9, 1889. [Google Scholar] [CrossRef]
- Prior, R.L.; Wu, X.; Schaich, K. Standardized Methods for the Determination of Antioxidant Capacity and Phenolics in Foods and Dietary Supplements. J. Agric. Food Chem. 2005, 53. [Google Scholar] [CrossRef]
- Orhan, I.; Şener, B.; Choudhary, M.I.; Khalid, A. Acetylcholinesterase and butyrylcholinesterase inhibitory activity of some Turkish medicinal plants. J. Ethnopharmacol. 2004, 91, 57–60. [Google Scholar] [CrossRef] [PubMed]
- Dudonné, S.; Vitrac, X.; Coutiére, P.; Woillez, M.; Mérillon, J.M. Comparative study of antioxidant properties and total phenolic content of 30 plant extracts of industrial interest using DPPH, ABTS, FRAP, SOD, and ORAC assays. J. Agric. Food Chem. 2009, 57, 1768–1774. [Google Scholar] [CrossRef] [PubMed]
- Crivineanu, M.; Durdun, C.; Nicorescu, I. Antioxidant activity of some polyphenolic extracts obtained from plants with antitumoral potential on linoleic acid emulsion. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2009, 66, 359–365. [Google Scholar]
- Fioravanti, D.E.; Schinella, G.R.; Tournier, H.A. Total antioxidant capacity and polyphenol content of 21 aqueous extracts obtained from native plants of Traslasierra valley (Argentina). Boletín Latinoamericano y del Caribe de Plantas Medicinales y Aromáticas 2009, 8, 529–539. [Google Scholar]
- Descallar, A.L.; Nunez., M.P.S.; Cabrera, M.L.N.; Martin, T.T.B.; Obemio, C.D.G.; Lanojan, R.S. Phytochemical Analysis and Antioxidant Capacity of Lycopodium clavatum Linn. from Lake Sebu, South Cotabato, Philippines. AIP Conf. Proc. 2017, 1803, 020021. [Google Scholar] [CrossRef]
- Brinza, I.; Abd-Alkhalek, A.M.; El-Raey, M.A.; Boiangiu, R.S.; Eldahshan, O.A.; Hritcu, L. Ameliorative Effects of Rhoifolin in Scopolamine-Induced Amnesic Zebrafish (Danio rerio) Model. Antioxidants 2020, 9, 580. [Google Scholar] [CrossRef]
- Stefanello, F.V.; Fontana, B.D.; Ziani, P.R.; Müller, T.E.; Mezzomo, N.J.; Rosemberg, D.B. Exploring Object Discrimination in Zebrafish: Behavioral Performance and Scopolamine-Induced Cognitive Deficits at Different Retention Intervals. Zebrafish 2019, 16, 370–378. [Google Scholar] [CrossRef]
- Konrath, E.L.; Neves, B.M.; Lunardi, P.S.; Passos, C.D.S.; Simões-Pires, A.; Ortega, M.G.; Gonalves, C.A.; Cabrera, J.L.; Moreira, J.C.F.; Henriques, A.T. Investigation of the in vitro and ex vivo acetylcholinesterase and antioxidant activities of traditionally used Lycopodium species from South America on alkaloid extracts. J. Ethnopharmacol. 2012, 139, 58–67. [Google Scholar] [CrossRef] [Green Version]
- Wang, B.; Guan, C.; Fu, Q. The Traditional Uses, Secondary Metabolites, and Pharmacology of Lycopodium Species. Phytochem. Rev. 2021, 1–79. [Google Scholar] [CrossRef]
- Tung, B.T.; Hai, N.T.; Thu, D.K. Antioxidant and acetylcholinesterase inhibitory activities in vitro of different fraction of Huperzia squarrosa (Forst.) Trevis extract and attenuation of scopolamine-induced cognitive impairment in mice. J. Ethnopharmacol. 2017, 198, 24–32. [Google Scholar] [CrossRef]
- Bailey, J.M.; Oliveri, A.N.; Levin, E.D. Pharmacological analyses of learning and memory in zebrafish (Danio rerio). Pharmacol. Biochem. Behav. 2015, 139, 103–111. [Google Scholar] [CrossRef] [Green Version]
- Dall’Acqua, S. Plant-derived acetylcholinesterase inhibitory alkaloids for the treatment of Alzheimer’s disease. Bot. Targets Ther. 2013, 3, 19–28. [Google Scholar] [CrossRef] [Green Version]
- Mukherjee, P.K.; Kumar, V.; Mal, M.; Houghton, P.J. Acetylcholinesterase inhibitors from plants. Phytomedicine 2007, 14, 289–300. [Google Scholar] [CrossRef] [PubMed]
- Cerros, R.; Mendoza-Ruiz, A.; Luisa Villarreal, M.; Pharm Sci, P.J.; Vázquez García, M.; Lino von Poser, G.; Apel, M.; Cerros Tlatilpa, R.; Luisa Villarreal, M.; Teresinha Henriques, A. Anticholinesterase activity and identification of huperzine A in three Mexican lycopods: Huperzia cuernavacensis, Huperzia dichotoma and Huperzia linifolia (Lycopodiaceae). Pak. J. Pharm. Sci. 2017, 30, 235–239. [Google Scholar]
- Kumar, H.; More, S.V.; Han, S.D.; Choi, J.Y.; Choi, D.K. Promising therapeutics with natural bioactive compounds for improving learning and memory—A review of randomized trials. Molecules 2012, 17, 10503–10539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, R.; Yan, H.; Tang, X.C. Progress in studies of huperzine A, a natural cholinesterase inhibitor from Chinese herbal medicine. Acta Pharmacol. Sin. 2006, 27, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bai, D.L.; Tang, X.C.; He, X.C. Huperzine a, a potential therapeutic agent for treatment of Alzheimer’s disease. Curr. Med. Chem. 2000, 7, 355–374. [Google Scholar] [CrossRef] [PubMed]
- Ohba, T.; Yoshino, Y.; Ishisaka, M.; Abe, N.; Tsuruma, K.; Shimazawa, M.; Oyama, M.; Tabira, T.; Hara, H. Japanese Huperzia serrata extract and the constituent, huperzine A, ameliorate the scopolamine-induced cognitive impairment in mice. Biosci. Biotechnol. Biochem. 2015, 79, 1838–1844. [Google Scholar] [CrossRef]
- Dumitru, G.; El-Nashar, H.A.S.; Mostafa, N.M.; Eldahshan, O.A.; Boiangiu, R.S.; Todirascu-Ciornea, E.; Hritcu, L.; Singab, A.N.B. Agathisflavone isolated from Schinus polygamus (Cav.) Cabrera leaves prevents scopolamine-induced memory impairment and brain oxidative stress in zebrafish (Danio rerio). Phytomedicine 2019, 58, 152889. [Google Scholar] [CrossRef]
- Wu, Q.; Gu, Y. Quantification of huperzine A in Huperzia serrata by HPLC-UV and identification of the major constituents in its alkaloid extracts by HPLC-DAD-MS-MS. J. Pharm. Biomed. Anal. 2006, 40, 993–998. [Google Scholar] [CrossRef]
- Feng, Y.N.; Zhang, X.F. Polysaccharide extracted from Huperzia serrata using response surface methodology and its biological activity. Int. J. Biol. Macromol. 2020, 157, 267–275. [Google Scholar] [CrossRef]
- Halldorsdottir, E.S.; Jaroszewski, J.W.; Olafsdottir, E.S. Acetylcholinesterase inhibitory activity of lycopodane-type alkaloids from the Icelandic Lycopodium annotinum ssp. alpestre. Phytochemistry 2010, 71, 149–157. [Google Scholar] [CrossRef] [PubMed]
- Rahimzadegan, M.; Soodi, M. Comparison of memory impairment and oxidative stress following single or repeated doses administration of scopolamine in rat hippocampus. Basic Clin. Neurosci. 2018, 9, 5–14. [Google Scholar] [CrossRef] [Green Version]
- Karthivashan, G.; Park, S.Y.; Kweon, M.H.; Kim, J.; Haque, M.E.; Cho, D.Y.; Kim, I.S.; Cho, E.A.; Ganesan, P.; Choi, D.K. Ameliorative potential of desalted Salicornia europaea L. extract in multifaceted Alzheimer’s-like scopolamine-induced amnesic mice model. Sci. Rep. 2018, 8, 8826. [Google Scholar] [CrossRef] [PubMed]
- Sharma, K. Cholinesterase inhibitors as Alzheimer’s therapeutics (Review). Mol. Med. Rep. 2019, 20, 1479–1487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trang, A.; Khandhar, P.B. Physiology, Acetylcholinesterase; StatPearls Publishing: Treasure Island, FL, USA, 2019. [Google Scholar]
- Orhan, I.; Özçelik, B.; Aslan, S.; Kartal, M.; Karaoglu, T.; Şener, B.; Terzioglu, S.; Iqbal Choudhary, M. In vitro biological activity screening of Lycopodium complanatum L. ssp. chamaecyparissus (A. Br.) Döll. Nat. Prod. Res. 2009, 23, 514–526. [Google Scholar] [CrossRef]
- Calderón, A.I.; Simithy-Williams, J.; Sanchez, R.; Espinosa, A.; Valdespino, I.; Gupta, M.P. Lycopodiaceae from panama: A new source of acetylcholinesterase inhibitors. Nat. Prod. Res. 2013, 27, 500–505. [Google Scholar] [CrossRef]
- Younus, H. Therapeutic potentials of superoxide dismutase. Int. J. Health Sci. 2018, 12, 88–93. [Google Scholar]
- Foyet, H.S.; Keugong Wado, E.; Ngatanko Abaissou, H.H.; Assongalem, E.A.; Eyong, O.K. Anticholinesterase and Antioxidant Potential of Hydromethanolic Extract of Ziziphus mucronata (Rhamnaceae) Leaves on Scopolamine-Induced Memory and Cognitive Dysfunctions in Mice. Evid.-Based Complement. Altern. Med. 2019, 2019, 4568401. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starke-Reed, P.E.; Oliver, C.N. Protein oxidation and proteolysis during aging and oxidative stress. Arch. Biochem. Biophys. 1989, 275, 559–567. [Google Scholar] [CrossRef]
- Ito, F.; Sono, Y.; Ito, T. Measurement and clinical significance of lipid peroxidation as a biomarker of oxidative stress: Oxidative stress in diabetes, atherosclerosis, and chronic inflammation. Antioxidants 2019, 8, 72. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Wang, J.; Zhou, G.S.; Tan, Y.J.; Tao, H.J.; Chen, J.Q.; Pu, Z.J.; Ma, J.Y.; She, W.; Kang, A.; et al. Studies of the Anti-amnesic Effects and Mechanisms of Single and Combined Use of Donepezil and Ginkgo Ketoester Tablet on Scopolamine-Induced Memory Impairment in Mice. Oxidative Med. Cell. Longev. 2019, 2019, 8636835. [Google Scholar] [CrossRef]
- Wen, C.; Zhang, J.; Zhang, H.; Dzah, C.S.; Zandile, M.; Duan, Y.; Ma, H.; Luo, X. Advances in ultrasound assisted extraction of bioactive compounds from cash crops—A review. Ultrason. Sonochem. 2018, 48. [Google Scholar] [CrossRef]
- Thaipong, K.; Boonprakob, U.; Crosby, K.; Cisneros-Zevallos, L.; Hawkins Byrne, D. Comparison of ABTS, DPPH, FRAP, and ORAC assays for estimating antioxidant activity from guava fruit extracts. J. Food Compos. Anal. 2006, 19, 669–675. [Google Scholar] [CrossRef]
- De Jong, N.; Emmer, M.; van Wamel, A.; Versluis, M. Ultrasonic characterization of ultrasound contrast agents. Med. Biol. Eng. Comput. 2009, 47. [Google Scholar] [CrossRef] [Green Version]
- Bhullar, K.S.; Rupasinghe, H.P.V. Polyphenols: Multipotent Therapeutic Agents in Neurodegenerative Diseases. Oxidative Med. Cell. Longev. 2013, 2013, 891748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szypuła, W.; Kiss, A.; Pietrosiuk, A.; Świst, M.; Danikiewicz, W.; Olszowska, O. Determination of huperzine a in Huperzia selago plants from wild population and obtained in in vitro culture by high-performance liquid chromatography using a chaotropic mobile phase. Acta Chromatogr. 2011, 23, 339–352. [Google Scholar] [CrossRef]
- Orhan, I.; Özçelik, B.; Aslan, S.; Kartal, M.; Karaoglu, T.; Şener, B.; Terzioglu, S.; Choudhary, M.I. Antioxidant and antimicrobial actions of the clubmoss Lycopodium clavatum L. Proc. Phytochem. Rev. 2007, 6, 189–196. [Google Scholar] [CrossRef]
- Zhang, Q.-W.; Lin, L.-G.; Ye, W.-C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, F.; Ghalib, R.; Sasikala, P.; Mueen Ahmed, K. Cholinesterase inhibitors from botanicals. Pharmacogn. Rev. 2013, 7, 121–130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mushtaq, G.; Greig, N.H.; Khan, J.A.; Kamal, M.A. Status of acetylcholinesterase and butyrylcholinesterase in Alzheimer’s disease and type 2 diabetes mellitus. CNS Neurol. Disord. Drug Targets 2014, 13, 1432–1439. [Google Scholar] [CrossRef]
- Borloz, A.; Marston, A.; Hostettmann, K. The determination of huperzine A in European Lycopodiaceae species by HPLC-UV-MS. Phytochem. Anal. 2006, 17, 332–336. [Google Scholar] [CrossRef]
- He, J.; Wu, X.-D.; Liu, F.; Liu, Y.-C.; Peng, L.-Y.; Zhao, Y.; Cheng, X.; Luo, H.-R.; Zhao, Q.-S. Lycopodine-Type Alkaloids from Lycopodium japonicum. Nat. Prod. Bioprospect. 2014, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Altemimi, A.; Lakhssassi, N.; Baharlouei, A.; Watson, D.; Lightfoot, D. Phytochemicals: Extraction, Isolation, and Identification of Bioactive Compounds from Plant Extracts. Plants 2017, 6, 42. [Google Scholar] [CrossRef]
- Hamilton, T.J.; Myggland, A.; Duperreault, E.; May, Z.; Gallup, J.; Powell, R.A.; Schalomon, M.; Digweed, S.M. Episodic-like memory in zebrafish. Anim. Cogn. 2016, 19, 1071–1079. [Google Scholar] [CrossRef] [PubMed]
- Egan, R.J.; Bergner, C.L.; Hart, P.C.; Cachat, J.M.; Canavello, P.R.; Elegante, M.F.; Elkhayat, S.I.; Bartels, B.K.; Tien, A.K.; Tien, D.H.; et al. Understanding behavioral and physiological phenotypes of stress and anxiety in zebrafish. Behav. Brain Res. 2009, 205, 38–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kalueff, A.V.; Stewart, A.M.; Gerlai, R. Zebrafish as an emerging model for studying complex brain disorders. Trends Pharmacol. Sci. 2014, 35, 63–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harborne, J.B. Phytochemical Methods: A Guide to Modern Techniques of Plant Analysis, 3rd ed.; Chapman and Hall: London, UK, 1998. [Google Scholar]
- Benzie, I.F.F.; Strain, J.J. The Ferric Reducing Ability of Plasma (FRAP) as a Measure of “Antioxidant Power”: The FRAP Assay. Anal. Biochem. 1996, 239. [Google Scholar] [CrossRef] [Green Version]
- Karadag, A.; Ozcelik, B.; Saner, S. Review of Methods to Determine Antioxidant Capacities. Food Anal. Methods 2009, 2. [Google Scholar] [CrossRef]
- Cao, G.; Alessio, H.M.; Cutler, R.G. Oxygen-radical absorbance capacity assay for antioxidants. Free Radic. Biol. Med. 1993, 14. [Google Scholar] [CrossRef] [Green Version]
- Ellman, G.L.; Courtney, K.D.; Andres, V.; Featherstone, R.M. A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem. Pharmacol. 1961, 7. [Google Scholar] [CrossRef]
- Sánchez-Rangel, J.C.; Benavides, J.; Heredia, J.B.; Cisneros-Zevallos, L.; Jacobo-Velázquez, D.A. The Folin–Ciocalteu assay revisited: Improvement of its specificity for total phenolic content determination. Anal. Methods 2013, 5. [Google Scholar] [CrossRef]
- Hanif, K.; Kumar, M.; Singh, N.; Shukla, R. Effect of homeopathic Lycopodium clavatum on memory functions and cerebral blood flow in memory-impaired rats. Homeopathy 2015, 104, 24–28. [Google Scholar] [CrossRef]
- Antunes, M.; Biala, G. The novel object recognition memory: Neurobiology, test procedure, and its modifications. Cogn. Process. 2012, 13, 93–110. [Google Scholar] [CrossRef] [Green Version]








| Phytoconstituent | Test | Result |
|---|---|---|
| Alkaloids | Dragendorff’s reagent | ++ |
| Steroids | Salkowski Test | + |
| Flavonoids | ||
| alpha benzopyrone | Wilstatter “Cyanidin” Test | + |
| leucoanthocyanins | Bate-Smith and Metcalf Method | − |
| Saponins | Froth test | − |
| Polyphenols | Ferric chloride Test | + |
| Sample a | DPPH Assay a IC50 (µg/mL) | FRAP Values a (mg AAE/g DM) | ABTS Assay a IC50 (µg/mL) | ORAC Assay a (µmol TE/g DM) | TPC a (mg GAE/g DM) | TFC a (mg QE/g DM) |
|---|---|---|---|---|---|---|
| L. selago | 84.33 ± 0.77 | 112.21 ± 2.03 | 12.13 ± 0.15 | 193.49 ± 1.52 | 9.21 ± 0.12 | 13.26 ± 0.02 |
| Ascorbic acid | - | 291.72 ± 2.90 | - | - | - | - |
| BHT | 92.41 ± 0.27 | 40.50 ± 0.19 | 19.32 ± 0.14 | - | - | - |
| AChE Inhibition % (1 mg/mL) a | BChE Inhibition % (1 mg/mL) a | |
|---|---|---|
| L. selago | 41 ± 1.21 | 68 ± 1.51 |
| Galantamine | 83 ± 1.63 | 72 ± 2.32 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valu, M.-V.; Ducu, C.; Moga, S.; Negrea, D.; Hritcu, L.; Boiangiu, R.S.; Vamanu, E.; Balseanu, T.A.; Carradori, S.; Soare, L.C. Effects of the Hydroethanolic Extract of Lycopodium selago L. on Scopolamine-Induced Memory Deficits in Zebrafish. Pharmaceuticals 2021, 14, 568. https://doi.org/10.3390/ph14060568
Valu M-V, Ducu C, Moga S, Negrea D, Hritcu L, Boiangiu RS, Vamanu E, Balseanu TA, Carradori S, Soare LC. Effects of the Hydroethanolic Extract of Lycopodium selago L. on Scopolamine-Induced Memory Deficits in Zebrafish. Pharmaceuticals. 2021; 14(6):568. https://doi.org/10.3390/ph14060568
Chicago/Turabian StyleValu, Mihai-Vlad, Catalin Ducu, Sorin Moga, Denis Negrea, Lucian Hritcu, Razvan Stefan Boiangiu, Emanuel Vamanu, Tudor Adrian Balseanu, Simone Carradori, and Liliana Cristina Soare. 2021. "Effects of the Hydroethanolic Extract of Lycopodium selago L. on Scopolamine-Induced Memory Deficits in Zebrafish" Pharmaceuticals 14, no. 6: 568. https://doi.org/10.3390/ph14060568