Phytochemicals, biological activity, and industrial application of lotus seedpod (Receptaculum Nelumbinis): A review
- 1School of Public Health and Health Management, Gannan Medical University, Ganzhou, China
- 2Scientific Research Center, Gannan Medical University, Ganzhou, China
- 3School of Basic Medical Sciences, Gannan Medical University, Ganzhou, China
- 4Key Laboratory of Environment and Health of Ganzhou, Gannan Medical University, Ganzhou, China
Lotus (Nelumbo nucifera Gaertn.) is a well-known food and medicinal plant. Lotus seedpod (Receptaculum Nelumbinis) is the by-products during lotus products processing, which is considered as waste. Numerous studies have been conducted on its phytochemicals, biological activity and industrial application. However, the information on lotus seedpod is scattered and has been rarely summarized. In this review, summaries on preparation and identification of phytochemicals, the biological activities of extracts and phytochemicals, and applications of raw material, extracts and phytochemicals for lotus seedpod were made. Meanwhile, the future study trend was proposed. Recent evidence indicated that lotus seedpods extracts, obtained by non-organic and organic solvents, possessed several activities, which were influenced by extraction solvents and methods. Lotus seedpods were rich in phytochemicals categorized as different chemical groups, such as proanthocyanidins, oligomeric procyanidins, flavonoids, alkaloids, terpenoids, etc. These phytochemicals exhibited various bioactivities, including ameliorating cognitive impairment, antioxidation, antibacterial, anti-glycative, neuroprotection, anti-tyrosinase and other activities. Raw material, extracts and phytochemicals of lotus seedpods could be utilized as sources for biochar and biomass material, in food industry and as dye. This review gives well-understanding on lotus seedpod, and provides theoretical basis for its future research and application.
Introduction
Nelumbo nucifera Gaertn. (also named as lotus), belonging to the mono-generic family Nelumbonaceae, is widely distributed in Asia, Americas and Oceania (1). Lotus has been cultivated as vegetable, functional food, and herb medicine for over 2,000 years (2). According to the phytomorphology, it can be divided into different parts, such as leaf, flower, stamen, rhizome, seed, seedpod and plumule (2). Almost every part can be used, and most of them are recorded in the “Chinese Pharmacopeia” (3). Among them, rhizome and seed are the main edible parts, which are popularly consumed as vegetables or functional foods due to their delicious taste and great nutritive and non-nutritive values (2).
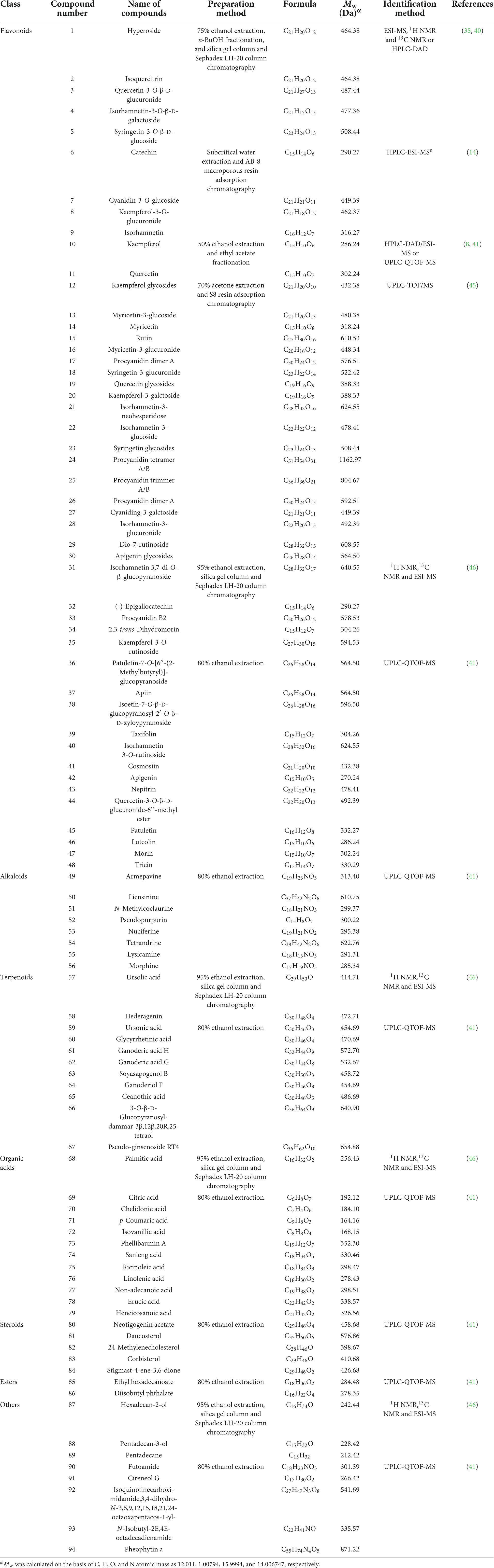
Lotus seedpod (Receptaculum Nelumbinis) is the mature receptacle of lotus house (seen in Figure 1), and is usually regarded as by-products during lotus seed processing. As one of the non-edible parts of lotus, its production is almost equivalent to the edible parts (4). In the Traditional Chinese medicine, lotus seedpod can be used for treating excessive menstrual bleeding and as hemostatic (5). According to the 2015 edition of “Chinese Pharmacopeia,” lotus seedpod charcoal (a processed product of lotus seedpod) has the usage in treating hemorrhage, urine blood, hemorrhoids bleeding, postpartum stasis, lochia, etc. However, this charcoal has gradually faded out of the market as the progress of medical science and technology. On account of lack adequate understanding on lotus seedpods, most of them are generally considered as wastes and are thrown away in the open or incinerated. This not only causes significant wastes of resources, but also brings heavy pollutions to environment. In this light, reusing the sources lotus seedpods into high value-added products is of great value.
Figure 1. Photographs of planted lotus (A), fresh lotus seedpod (B) and sun-dried lotus seedpod (C) taken by Jian-Lin Shen in August, 2022.
In the past decades, more and more attention has been paid to phytochemicals, biological activity and industrial application of lotus seedpods. A variety of phytochemicals have been derived and identified from lotus seedpods, including proanthocyanidins (6), oligomeric procyanidins (7), polyphenols (8), flavonoids and others (9). Numerous studies have demonstrated that extracts and phytochemicals of lotus seedpods possessed various biological activities, such as ameliorating cognitive impairment (10–12), antioxidation (8, 9, 13, 14), antibacterial (15–17), anti-glycative (7, 18, 19) and anti-diabetes (20). Due to the good biological activities and superior physicochemical property, raw material, extracts and phytochemicals of lotus seedpods have been increasingly applied in food and other industries (21–23). Despite of multiple researches have been carried out to lotus seedpods, the information concerning it was scattered. In contrast, summary on other parts of lotus like seeds (1, 24) and leaf (25) have been reported. However, the review on lotus seedpods is rare.
Hence, in this article, the information on phytochemicals, biological activity and industrial application of lotus seedpods was systematically summarized. Meanwhile, the future study trend about lotus seedpods was proposed. This work gives well-understanding on lotus seedpod, and provides theoretical basis for research as well as exploitation and application of it, which is helpful to the protection of lotus seedpod sources.
Preparation and identification of phytochemicals from lotus seedpods
Extracts of lotus seedpods can be mainly obtained by water (9, 14, 26–29) and organic (ethanol, methanol, glycerol, n-hexane, chloroform, ethyl acetate, butanol, acetone-water, etc.) (8, 13, 29–37) solvents. However, these traditional organic solvents methods have the limitations as low efficiency, low yield and potential environmental hazards, and water is not effective in extracting moderately polar and non-polar compounds (14). Subcritical water might be a good choice to overcome the drawback of water extraction. Anyway, water and ethanol are widely used as the extraction solvents. Chemical component analysis indicated that contents of total phenolics, flavonoids and proanthocyanidins in lotus seedpods extracts were in the range of 32.1∼607.6, 42.8∼ 862.7 and 10.6∼331.0 mg/g extract, respectively (35). In order to increase the recovery rate of phenolic compounds from lotus seedpod, ultrasound- and gas-assisted extraction methods can be applied (13, 36). Among them, the proanthocyanidins can be sequentially extracted by organic reagent like ethyl acetate to obtain the oligomeric procyanidins, which includes 10.9% catechin, 9.1% epicatechin, 53.6% dimer, 19.5% trimer and 1.9% tetramer (38, 39).
Before phytochemicals identification, the above-mentioned lotus seedpods extracts are usually purified by organic solvent (n-BuOH or ethyl acetate) fractionation (8, 35, 39–44), column (silica gel column, AB-8 macroporous resin or S8 resin column) adsorption (14, 35, 39, 40, 42–47), and Sephadex LH-20 column chromatography (35, 40, 46, 47). The lotus seedpods extracts before and after purification can be identified by electrospray ionization-mass spectrometry (ESI-MS) (34, 35, 46), nuclear magnetic resonance spectrometer (NMR) (34, 35, 47, 48), high-performance liquid chromatography (HPLC)/electrospray ionization tandem mass spectrometry (ESI-MS-MS) (9), HPLC-diode array detector (DAD)-ESI-MS (8), HPLC-ESI-MS (14, 49), LC-MS (38, 44), HPLC-DAD (40), HPLC-DAD-MS (50), ultra-performance liquid chromatography triple-time of flight/MS (UPLC-TOF/MS) (45, 51) and/or UPLC-quadrupole (Q) TOF-MS (41) methods. However, the analyses of phytochemicals might be limited by the capacity of the identification database, as above-mentioned detection methods depended deeply on standard secondary spectra database (41). Having considerding that used standards and the compounds in the database are limited, the completeness and accuracy of identification of phytochemicals by these methods are a little insufficient. The combination of targeted/untargeted metabolomics analysis and comparison with standards might be applied to improve this.
Up to date, more than 94 compounds have been well-identified from lotus seedpods, as summarized in Table 1. Except the most reported two phytochemicals (proanthocyanidins and oligomeric procyanidins), the identified compounds can be generally classified into flavonoids, alkaloids, terpenoids, organic acids, steroids, esters and others (8, 14, 35, 40, 41, 45, 46). According to Table 1, the flavonoids are mostly identified in lotus seedpods. Meanwhile, Lee et al. (9) have found that the contents of 8 flavonoids included myricetin-3-galactoside, quercetin-3-glucuronide, isoquercitrin, isorhamnetin-3-glucuronide, isorhamnetin-3-glucoside, quercetin, kaempferol and isorhamnetin, were 11.52, 122.44, 29.44, 30.27, 29.73, 0.42, 2.01, and 0.80 mg/100 g respectively in lotus seedpod water extracts. Moreover, chemical structures of some compounds identified in lotus seedpods have been proposed, as illustrated in Figure 2.
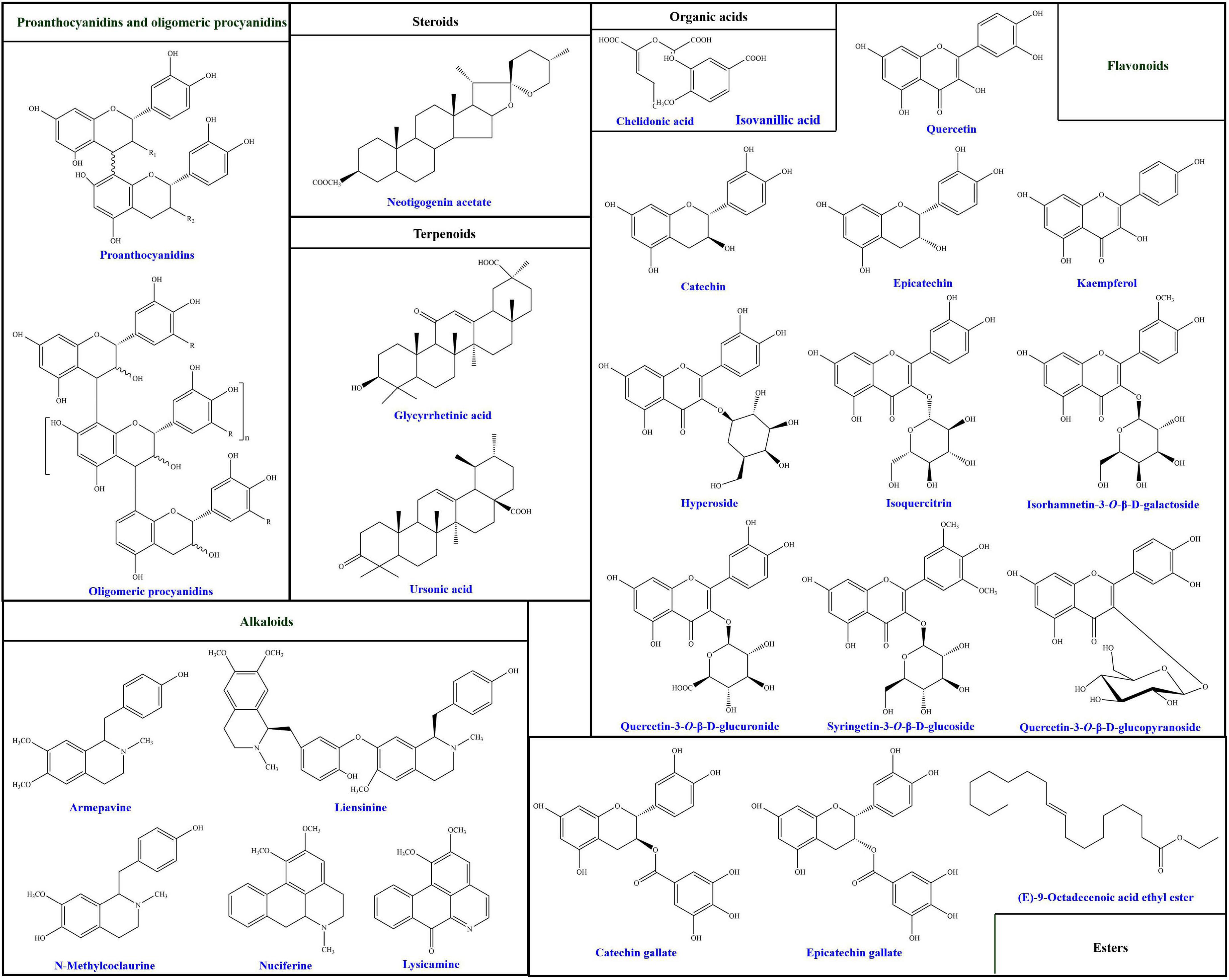
Figure 2. Chemical structures of some compounds identified in lotus seedpods. These chemical structures are redrawn on the basis of previous studies (8, 34, 35, 38, 39, 41, 44, 47).
Different preparation methods produced differences in the chemical structures of phytochemicals from lotus seedpods. In the study taken by Wu et al. (35), five compounds have been respectively obtained from n-BuOH extracts of lotus seedpods by silica gel column chromatography with different CH2Cl2-MeOH elution ratios and Sephadex LH-20 column chromatography. These five compounds have different chemical structures, which have been identified to be hyperoside, isoquercitrin, quercetin-3-O-β-D-glucuronide, isorhamnetin-3-O-β-D-galactoside and syringetin-3-O-β-D-glucoside. On the other hand, extracts of lotus seedpods from different production places have been found to contain different chemical compositions. Another study of Wu et al. (40) has indicated that 50% ethanols of 20 lotus seedpods samples, collected from different regions (Fujian, Jiangxi, Zhejiang, Beijing, Jiangsu, Hubei, Hebei, and Hebei) of China, showed different contents of these five compounds. An investigation conducted by Liu et al. (50) has demonstrated that hyperoside and isoquercitrin in methanol extracts of 11 lotus seedpods samples, from different localities of Jianning County, Fujian province, China, exhibited different chemical fingerprints. Otherwise, different sources of lotus seedpods also contained different chemical compositions. The research of Limwachiranon et al. (45) has implied that lotus seedpods from three commonly consumed cultivars (Shilihehua, Jianlian and liyebailian) showed differences in phenolic, flavonoid, and proanthocyanidins contents.
Biological activity of lotus seedpods extracts
Lotus seedpods extracts have been demonstrated to possess antioxidation (8, 9, 13, 14, 29, 34–36), anti-cancer (8, 14, 32), anti-melanogenic (27, 33), anti-inflammatory (28, 31), anti-irradiation (37), cardioprotection (30) and hepatoprotection (26) bioactivities, as displayed in Figure 3A and Supplementary Table 1.
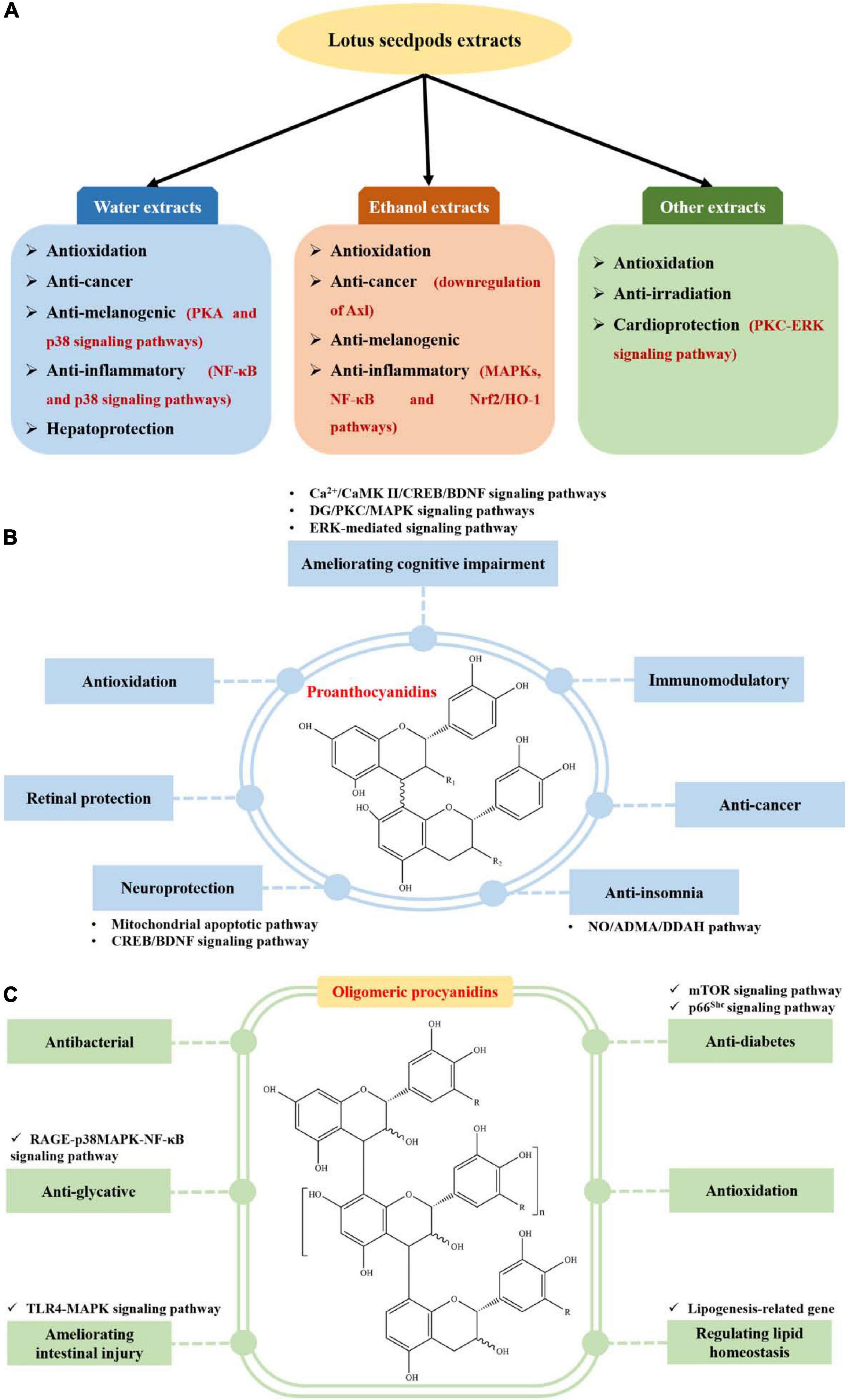
Figure 3. Biological activities of extracts (A), proanthocyanidins (B), and oligomeric procyanidin (C) from lotus seedpods.
Biological activity of water extracts from lotus seedpods
Water extracts from lotus seedpods have been proven to exert antioxidation (9, 14, 29), anti-cancer (14), anti-melanogenic (27), anti-inflammatory (28) and hepatoprotection (26) activities.
In terms of the antioxidation activity, chemical assays have indicated that water extracts from lotus seedpods had scavenging effects on DPPH, ABTS and NO2– radicals, and ferric reducing ability (14, 29). A cell experiment has demonstrated that the extracts could dose-dependently improved the survival and function of rat pancreatic RIN-m5F cells induced by H2O2 through down-regulation of apoptosis and up-regulation of autophagy, revealing as increasing protein expressions of LC3II, Atg5/12, p62, class III PI3K, Beclin-1 and p-Bad/Bad, and decreasing protein expressions of active-caspase-3 and cleavage PARP-1 along with Bax/Bcl-2 ratio (9). Regarding to anti-melanogenic effect, water extracts of lotus seedpods could inhibit melanin synthesis in α-melanocyte stimulating hormone-induced B16F10 cells and in UVB-induced mice, which involved both PKA and p38 signaling pathways (27). In the cell experiment, the extracts decreased protein expressions of p-PKA/PKA, p-p38/p38, p-CREB/CREB and MITF, and protein and mRNA expressions of tyrosinase, TRP-1 and MC1R of B16F10 cells. In the animal experiment, the extracts reduced protein expressions of tyrosinase, TRP-1, TRP-2, p-PKA/PKA and p-p38/p38 in ears of mice. As to anti-inflammatory action, water extracts of lotus seedpods exerted protective effects against LPS-induced HepG2 cells and LPS-induced mice via NF-κB and p38 signaling pathways (28). In the cell experiment, the extracts could lessen mRNA expressions of IL-6, COX-2 and iNOS, and protein expressions of COX-2, iNOS, NF-κB, IKK, p-IκB/IκB, p-p38/p38, TLR4 and MyD88 of HepG2 cells. In the animal experiment, the extracts could decrease protein expressions of COX-2, iNOS, NF-κB, IKK, p-IκB/IκB and p-p38/p38 in liver of mice. To hepatoprotection effect, water extracts of lotus seedpods reduced lipid accumulation and lipotoxicity in oleic acid-induced HepG2 cells through anti-apoptotic and anti-autophagy mechanisms, showing as adding Bcl-2 protein expression, and lowering protein expressions of LC3-II/LC3-I, Atg5/12, active-caspase-3/8/9, cleaved PARP, Bax and mitochondrial membrane depolarization, as well as Bax/Bcl-2 ratio (26). Additionally, water extract from lotus seedpods had anti-proliferation effect on HepG2 cells (14).
On the other hand, subcritical water extracts from lotus seedpods have been determined to reveal antioxidation activity, showing as scavenging DPPH, ABTS and NO2– radicals and ferric reducing ability, and anti-cancer effect against cell proliferation of HepG2 cells (14).
Biological activity of ethanol extracts from lotus seedpods
Ethanol extracts from lotus seedpods have been indicated to exhibit antioxidation (8, 29, 34, 35), anti-cancer (8, 32), anti-melanogenic (33) and anti-inflammatory (31) actions.
With regard to antioxidation effect, chemical assays have demonstrated that ethanol extracts from lotus seedpods exhibited scavenging effects on DPPH, ABTS, OH, O2– and H2O2 radicals, ferric reducing ability and metal ion chelating activity (8, 29, 34, 35). A cell experiment has shown that the ethanol extracts revealed cytoprotection on H2O2-induced RAW264.7 cells and reduced MDA level (34). In terms of anti-cancer activity, ethanol extracts from lotus seedpods exerted anti-proliferation effect on HepG2, LNcap, A549 and H460 cells (8, 32). Thereinto, the extracts induced cell apoptosis of A549 and H460 cells via downregulation of Axl, illustrating as increase of cleavage PARP and γ-H2AX protein expressions, and decrease of PARP protein expression as well as Axl protein and mRNA expressions (32). Regarding to anti-melanogenic effect, inhibition of the extract on melanin synthesis in α-melanocyte stimulating hormone-induced B16F10 cells was related to downregulation of activity and protein expression of tyrosinase (33). As to anti-inflammatory action, MAPKs, NF-κB and Nrf2/HO-1 pathways were regulated by the extracts to LPS-induced RAW264.7 cells, which showing as increasing protein expressions of Nrf2 and HO-1, and decreasing protein expressions of iNOS, COX-2, p-p38/p38, p-ERK/ERK, p-JNK/JNK, p65 and p-p65 (31).
Biological activity of other extracts from lotus seedpods
Methanol, glycerol, n-hexane, chloroform, ethyl acetate, butanol and acetone-water extracts from lotus seedpods have been shown to possess antioxidation (13, 29, 36), anti-irradiation (37) and cardioprotection effects (30). The extracts obtained by these solvents (except acetone-water) exerted antioxidation activities in scavenging DPPH and ABTS radicals and ferric reducing ability (13, 29, 36). Acetone-water extract of lotus seedpods showed anti-irradiation effect against 60Co irradiation-induced mice, behaving as increments of survival time, activities of SOD, CAT and GPX in liver, levels of white blood cells, red blood cells, platelets and hemoglobin, and spleen weight and index, and reduction of LPO level in liver and chromosomal aberrations in the bone marrow, to mice (37). 100% methanol extract had cardioprotection effect on Ang II-induced H9c2 cells through suppression of PKC-ERK signaling pathways, which exhibiting as declinations of protein expressions of NFATc-1, ANP, BNP, MLC2, NOX2, NOX4, p-NF-κB/NF-κB, AT1R, RAGE, PKC, p-PKC and p-ERK1/2 (30).
Comparison on biological activities of different extracts from lotus seedpods
Extracts from lotus seedpods acquired with different solvents and methods had distinctions in biological activities. Antioxidation activities of 80% ethanol, n-hexane, chloroform, ethyl acetate, butanol and water extracts from lotus seedpods have been investigated in the study of Kim and Shin (29). The results suggested that these extracts had different flavonoid and proanthocyanidin contents, and the scavenging activities on DPPH and ABTS radicals and ferric reducing ability for them were 94.319, 33.387, 85.263, 76.099, 93.944% and 94.587, 92.937, 9.781, 93.940, 85.755, 93.184% and 93.184%, and 1.551, 0.410, 0.905, 1.099, 1.431, and 1.448 respectively, at the concentration of 0.8 mg/mL. The findings of Yan et al. (14) have manifested that subcritical water extract (SWE) and hot water extract (HWE) from lotus seedpods contained different polyphenol (815.4 and 785.6 mg GAE/g DW, respectively) and flavonoid (1012.05 and 932.56 mg RE/g DW) contents. The SWE showed significantly higher scavenging activities of DPPH, ABTS and NO2– radicals and ferric reducing ability, along with antiproliferative activity on HepG2 cells, as compared to the HWE.
Moreover, the study taken by Bao et al. (36) has made comparisons on the antioxidant activities of extracts from lotus seedpods by four extraction means, including ultrasonic coupled with glycerol (UG), ultrasonic using water (UW), water bath incubation with glycerol (WG) and water bath incubation using water (WW). The results displayed that the extract gained by UG had the relatively highest scavenging activities on DPPH and ABTS radicals and ferric reducing ability. Another investigation of them implied that extract from lotus seedpods obtained by gas-assisted combined with glycerol approach exhibited obvious higher scavenging effets on DPPH and ABTS radicals along with ferric reducing ability than that acquired by WG (13).
Biological activity of phytochemicals from lotus seedpods
Phytochemicals from lotus seedpods have been determined to reveal a variety of biological activities, including ameliorating cognitive impairment (10–12, 52–61), antioxidation (4, 5, 62–64), antibacterial (15–17, 65), anti-glycative (7, 18, 19), neuroprotection (48, 66), anti-tyrosinase (46, 47), retinal protection (6), anti-insomnia (67), anti-cancer (68), immunomodulatory (69), ameliorating intestinal injury (70), anti-diabetes (20), regulating lipid homeostasis (38), anti-inflammatory (71) and α-glucosidase inhibitory (4) activities.
Biological activity of proanthocyanidins from lotus seedpods
Recently, proanthocyanidin is one of the mostly investigated phytochemicals from lotus seedpods. They have been implied to possess ameliorating cognitive impairment (11, 12, 52–61), antioxidation (62–64), neuroprotection (10, 48, 66), retinal protection (6), anti-insomnia (67), anti-cancer (68) and immunomodulatory (69) activities, as displayed in Figure 3B and Supplementary Table 1.
Animal studies have indicated that proanthocyanidins from lotus seedpods ameliorated cognitive impairment of D-galactose- (12), extremely low frequency electromagnetic fields- (11, 56, 57), scopolamine- (52–55) or alcohol-induced mice (52), senescence-accelerated mice (61), and aged rats (58–60). In terms of D-galactose-induced mice model, the ameliorating cognitive impairment action of the proanthocyanidins correlated with reverse of oxidative damage, prevention of Aβ overproduction and suppression of NO production, appearing as enhancements of SOD and GPX activities in brain, reductions of Aβ1–42, NO and MDA levels and AchE, MAO-B, tNOS and nNOS activities in brain, and declinations of nerve cell apoptosis and p53 protein expression in hippocampus (12). Regarding to extremely low frequency electromagnetic fields-induced mice model, Ca2+/CaMK II/CREB/BDNF and DG/PKC/MAPK signaling pathways involved in the ameliorating cognitive impairment effect of the proanthocyanidins (11, 57). The proanthocyanidins increased protein expressions of CaMKII, PKCα, BDNF and p-ERK1/2, and decreased concentrations of Ca2+, IP3, DAG, glutamate, GABA and [Ca2+]i and protein expressions of Gi, PKA, PKCβ, PP2B, ASK1, NR2B, p-CREB and p-JNK1/2, in hippocampus of mice. Moreover, the action was also related to improvement of antioxidant status, showing as increasing activities of SOD, CAT and GPX, and decreasing levels of MDA and NO and activity of NOS, in serum and hippocampus of mice (56). As to scopolamine- or alcohol-induced mice model, the ameliorating cognitive impairment action of the proanthocyanidins was correalted with improvement of antioxidant ability and cholinergic activity, exhibiting as elevating T-AOC level and activities of SOD and GPX, lessening levels of MDA, MAO-B, AchE and NO, activities of MPO, AchE, tNOS, nNOS and iNOS, and nNOS mRNA expression, in hippocampus, brain, serum and/or colon (52–55). For senescence-accelerated mice model, the ameliorating cognitive impairment effect of the proanthocyanidins was in connection with boost of antioxidant level, reflecting as enhancing GSH level and SOD and GPX activities, and reducing NO and MDA levels and nNOS and total NOS activities in brain and/or serum (61). To aged rats model, the ameliorating cognitive impairment effect of the proanthocyanidins correlated with changes of NO system (60), activation of hippocampal CREB through ERK-mediated signaling pathway (59) and rejuvenation of antioxidant and cholinergic systems (58). The proanthocyanidins elevated levels of GSH, T-AOC, AchE and Ach, activities of CAT and GPX, protein expressions of p-CREB, BDNF, p-ERK42/ERK42, p-ERK44/ERK44 and iNOS, and mRNA expressions of iNOS and BDNF, and declined levels of NO and MDA and activities of tNOS and iNOS in hippocampus and/or cerebral cortex of rats.
A chemical assay has shown that proanthocyanidins of lotus seedpods exhibited antioxidation activity in scavenging DPPH and ABTS radicals along with ferric reducing ability (62). A further cell experiment has suggested that the proanthocyanidins could relieve oxidative damage in H2O2-induced HUVECs, appearing as increasing activities of SOD and GPX and production of NO, and decreasing levels of MDA and ET-1 (62). Animal experiments have proven that the proanthocyanidins exerted antioxidation action in aged rats (64) and extremely low frequency electromagnetic fields-induced mice (63). In terms of aged rats model, the proanthocyanidins promoted the activities of SOD, CAT and GPX and level of GSH, and lessened TBARS content, in serum, heart, liver, kidney, lung or muscle (64). Regarding to extremely low frequency electromagnetic fields-induced mice model, the proanthocyanidins aggrandized activities of SOD, CAT, GPX, GR and GST, and lowered MDA level, in serum and cerebral cortex (63).
Cell experiments have revealed that proanthocyanidins of lotus seedpods possessed neuroprotection activity against amyloid-β-induced PC12 cells (10), extremely low frequency electromagnetic fields-induced primary cultured rat hippocampal neurons (66) and methyl mercuric chloride-induced neuron/astrocyte co-cultured cells (48). The activity was realized through inhibitions of oxidative stress and mitochondrial apoptotic pathway, and activation of CREB/BDNF signaling pathway. The proanthocyanidins raised levels of GSH, T-AOC and mitochondrial membrane potential, activities of SOD and GPX, protein expressions of p-CREB/CREB, BDNF, p-AKT/AKT, p-ERK/ERK, Bcl-xl, Bcl-2, SOD1/SOD2, Bcl-xl, Nrf2, HO-1, nuclear Nrf2, β-III-Tubulin, SYN and Arc and mRNA expression of BDNF, and down-regulated concentrations of LDH, MDA, Ca2+ and ROS, and protein expressions of Bad, Bax and caspase-3/-9 along with Bax/Bcl-2 ratio (10, 48, 66).
Animal experiment has demonstrated that proanthocyanidins of lotus seedpods reflected retinal protection against light exposure-induced rats through anti-oxidative stress, anti-apoptosis and neuroprotective effects, displaying as improving activities of SOD and GPX and mRNA and protein expressions of Bcl-2, and lowering retinal apoptosis, mRNA expressions of caspase-3, p53 and Bax, and protein expressions of pro-caspase-3, cleaved caspase-3, p53 and Bax (6). The proanthocyanidins exerted anti-insomnia effect to rats by regulating NO/ADMA/DDAH pathway, showing as increasing levels of 5-HT, GABA and NO, and protein and mRNA expressions of DDAH1, DDAH2 and nNOS, and decreasing levels of NE, Glu, ADMA and 8-isoprostane, in brain (67). Proanthocyanidins from lotus seedpods exhibited immunomodulatory effect against extremely low frequency electromagnetic fields-induced mice, and increased protein expressions of IL-2, IL-6, IL-10, INF-γ and Bcl-xl, and DNA contents, and decreased protein expressions of TNF-α and caspase-3/9 along with Bax/Bcl-2 ratio and apoptotic splenocytes in spleen (69). Otherwise, cell experiment has showed that proanthocyanidins from lotus seedpods possessed anti-cancer activity in HepG2 cells through inducing autophagy and ROS generation (68).
In addition, there are some connects between the different bioactivities of proanthocyanidins from lotus seedpods. The retinal protection of proanthocyanidins against light exposure-induced rats was realized partly through their antioxidation and neuroprotection activities (6). Ameliorating cognitive impairment action of proanthocyanidins was reported to be related to their antioxidation effects in D- galactose-, scopolamine-, alcohol and scopolamine, senescence-accelerated or extremely low frequency electromagnetic field exposure-induced mice (12, 52–54, 56, 61) as well as cognitively impaired aged rats (58). The neuroprotection of proanthocyanidins was associated with their antioxidation action in extremely low frequency electromagnetic field-induced primary cultured hippocampal neurons (66) and methyl mercuric chloride-induced neuron/astrocyte co-cultured cells (48).
Biological activity of oligomeric procyanidins from lotus seedpods
Oligomeric procyanidin is another one of the mostly studied phytochemicals from lotus seedpods. They have been demonstrated to show antibacterial (15–17, 65), anti-glycative (7, 18, 19), ameliorating intestinal injury (70), anti-diabetes (20), antioxidation (5) and regulating lipid homeostasis (38) activities, as indicated in Figure 3C and Supplementary Table 1.
Oligomeric procyanidins of lotus seedpods showed antibacterial activity in vitro and in vivo. The oligomeric procyanidins had the minimal inhibitory concentrations on Escherichia coli K88ac, F18ac, 10899 and BL21 as 0.80, 1.20, 1.25, and 1.25 mg/mL, respectively (17, 65). Moreover, synergistic effect was observed between the oligomeric procyanidins and water-soluble Poria cocos polysaccharides or carboxymethyl pachyman in inhibitory effect on Escherichia coli (16, 17). The antibacterial mechanism of oligomeric procyanidins on Escherichia coli has been disclosed to be increments of extracellular alkaline phosphatase, ROS production, activities of SOD and CAT and mRNA expressions of sodA, soxR, oxyR and oxyS (15). Further study has indicated that the oligomeric procyanidins reflected antibacterial activity against high-lactose diet-induced mice by enhancing abundances of Lactobacillus and Bifidobacterium, and lessening populations of Escherichia coli and Enterococcus in feces (15). On the other hand, the oligomeric procyanidins ameliorated intestinal injury against enterotoxigenic Escherichia coli-infected diarrhea mice (70). This effect was related to the modulation of TLR4-MAPK signaling pathway, reflecting as increasing protein and mRNA expressions of ZO-1, claudin-1 and occludin, and decreasing protein expressions of p-p38, p-JNK1/2 and p-ERK1/2 and RNA expressions of TNF-α, IL-8, IL-1β, IL-6, CD14, TLR4, p38 and NF-κB in jejunum/ileum.
Oligomeric procyanidins from lotus seedpods exerted anti-glycative activity on Caco-2 cells treated with digestive fluid (19), high-AGEs diet-induced mice (7) and high-fat diet-induced rats (18). RAGE-p38MAPK-NF-κB signaling pathway was involved in their activities. In the animal experiments, the oligomeric procyanidins decreased protein expressions of NF-κB, p38MAPK, p-p38MAPK, RAGE and p65NF-κB, and mRNA expressions of TNF-α, IL-6, NADPH, COX and RAGE in liver of mice or rats induced by high-AGEs diet or high-fat diet (7, 18). In the cell experiment, the oligomeric procyanidins reduced protein expressions of RAGE, p-p38MAPK, p65NF-κB and p-p65NF-κB, and mRNA expressions of NADPH, TNF-α, IL-6, ICAM-1 and VCAM-1 of Caco-2 cells treated with digestive fluid (19). Moreover, absorption and metabolism of oligomeric procyanidins from lotus seedpods have been investigated in the study of Wu et al. (72). Eight metabolites, including (+)-catechin, caffeic acid, syringic acid, 3-hydroxybenzoic acid, 3-hydroxyphenylacetic acid, 3-hydroxyphenylpropionic acid, ferulic acid and m-coumaric acid, have been detected in in urine of rats after 24 h post-administration of 300 mg/kg body weight of the oligomeric procyanidins. Among them, (+)-catechin had much better inhibition activity on AGE formation and methylglyoxal scavenging effect, while syringic acid showed the best scavenging ability on DPPH radical. Furthermore, Wu et al. (49) have compared the inhibitory effects of oligomeric procyanidin from lotus seedpods and its three main monomers [(+)-catechin, (-)-epicatechin and (-)-epigallocatechin gallate] on releases of AGE and CML formation in simulated gastrointestinal digestion, for studying the structure-activity relationship. The results indicated that (-)-epigallocatechin gallate exhibited the strongest activities.
Animal experiment has shown that oligomeric procyanidins from lotus seedpods possessed anti-diabetes action against streptozotocin-induced mice by attenuating mTOR signaling and enhancing glucose homeostasis (20). This action was related to enhancement of protein expressions of GLUT2, GK, p-AKT, UCP-1 and GLUT4 and mRNA expressions of HK II, PFK and PK, and reduction of protein expressions of mTOR, p66Shc, PKCβ, FoxO1a, p-FoxO1a and GLUT1 and mRNA expressions of PEPCK, G-6-Pase, SREBP-1c, ACL, ACC1, FAS, SCD1 and S6K1, in liver, skeletal muscle, white adipose tissue and/or brown adipose tissue. Chemical assays have revealed that the oligomeric procyanidins had antioxidation action in scavenging ⋅OH, O2– and H2O2 radicals (5). Animal investigation has suggested that the oligomeric procyanidins regulated the lipid profile of high fat/sucrose diet-induced rats by suppressing the lipogenesis-related gene expressions, such as SREBP-1c, FAS, ACC1, PPARγ and CD36, and elevating phase II drug metabolism enzyme SULT2B1b gene expression (38).
Additionally, some connects between the different bioactivities of oligomeric procyanidin could be found. Wu et al. (7, 18, 19, 72) have argued that anti-glycative activity of oligomeric procyanidins from lotus seedpods is positively correlated to their antioxidant capacities. The study taken by Tang et al. (70) has indicated that the antioxidant mechanism involved the ameliorating intestinal injury activity of oligomeric procyanidins from lotus seedpods on Enterotoxigenic Escherichia coli infected diarrhea mice.
Biological activity of others phytochemicals from lotus seedpods
Others phytochemicals like β-sitosterol, quercetin, kaempferol and polysaccharides from lotus seedpods have been indicated to have anti-tyrosinase (46, 47), anti-inflammatory (71), antioxidation (4) and α-glucosidase inhibitory (4) effects. β-sitosterol, quercetin 3-O-β-D-galactopyranoside and kaempferol 3-O-β-D-glucopyranoside from lotus seedpods have been determined to be tyrosinase inhibitors (46, 47) as shown in Supplementary Table 1. A (E)-9-Octadecenoic acid ethyl ester from lotus seedpods showed anti-inflammatory effect against LPS-induced RAW264.7 cells, and the effect was realized through regulating MAPKs and NF-κB signaling pathways, which displaying as up-regulations of NF-κB nuclear translocation, protein expressions of ERK, p38 and JNK, and protein and mRNA expressions of iNOS and COX2 (71). Flavonol glycosides fractionated from extracts of lotus seedpods, namely hyperoside, isoquercitrin, quercetin-3-O-β-D-glucuronide, isorhamnetin-3-O-β-D-galactoside and syringetin-3-O-β-D-glucoside, appeared scavenging activities on ABTS and DPPH radicals (35). Our previous study has indicated that water-extracted polysaccharides from dried lotus seedpods possessed good scavenging effects on ABTS, DPPH and ⋅OH radicals, and an obvious inhibitory effect on α-glucosidase activity (4).
Applications of raw material, extracts and phytochemicals of lotus seedpods
Applications of lotus seedpods
Lotus seedpods can be applied as sources for biochar (21, 73–76) and biomass material (22, 77–80).
As a source of biochar, lotus seedpods can be used to constitute sensor (74), absorber (21, 73, 75, 81) and detector (76). A portable, flexible, outdoor and inexpensive sensing platform for hyperin has been established by lotus seedpods biochar and molybdenum disulfide, using a green co-hydrothermal approach (74). Lotus seedpod-derived biochar can be used for producing available and effective biosorbents for cadmium (73), methylene blue (81) and 17 β-estradiol (21), along with Co3O4 microwave absorbent (75). Otherwise, a carbon quantum dots was synthesized on the basis of lotus seedpod by hydrothermal synthesis method, which could be utilized for Fe(III) detection (76).
In terms of using for biomass material, lotus seedpod is high-stable electrode material for supercapacitors (22, 78, 80). Meanwhile, lotus seedpod-derived hard carbon with hierarchical porous structure is a stable anode for sodium-ion batteries (79). Otherwise, an efficient metal-free catalyst derived from lotus seedpod exhibited excellent oxygen reduction reaction (77).
Applications of lotus seedpods extracts and phytochemicals
Lotus seedpod extracts (8, 82), proanthocyanidin (83, 84) and oligomeric procyanidin (5, 19, 23, 43, 44, 49, 72, 85, 86) can be used in food industry. Lotus seedpod extracts can inhibit lipid oxidation. 80% ethanol extract and water extract reduced the acid value, peroxide value and TBARS level of lard (82). 50% ethanol extract decreased the peroxidation level of linoleic acid (8). Moreover, conjugate complexes produced by lotus seedpod proanthocyanin and whey protein have potential applications in emulsions (83, 84). Lotus seedpod proanthocyanidin-whey protein complexes improved the chemical stability of β-carotene nanoemulsions (84). Lotus seedpod proanthocyanidin was grafted to whey protein isolate for creating nature-derived antioxidant emulsifiers, which had good DPPH radical scavenging activity and ferric reducing ability (83).
Inhibition of AGEs formation is an important application for lotus seedpod oligomeric procyanidin in food systems, such as bovine serum albumin-glucose (43, 72), lactose-lysine (44) and yogurt (86) systems. Meanwhile, addition of lotus seedpod oligomeric procyanidin increased the growth of Lactobacillus plantarum, titratable acidity, DPPH scavenging effect, solid-like properties, hardness, adhesiveness, gumminess and chewiness of yogurt (86). Moreover, lotus seedpod oligomeric procyanidin and its three monomers including catechin, epicatechin and epigallocatechin gallate could inhibit AGEs release from glycated casein during gastrointestinal digestion (49). At the same time, lotus seedpod oligomeric procyanidin and catechin could also enhance the scavenging effects of digestive fluid on DPPH, OH and ABTS radicals and its ferric reducing ability (19). On the other hand, lotus seedpod oligomeric procyanidin is beneficial to the storage and process of food. Lotus seedpod oligomeric procyanidin could decline the peroxide values of lard or soybean oil (5). Lotus seedpod oligomeric procyanidin possessed inhibitory effect on the retrogradation property of rice starch (23).
Beside, oligomeric procyanidin extracted from lotus seedpod has been used to dye the tussah silk fabric (87).
Conclusion and perspectives
Lotus seedpod is a promising food and medicine source. Extracts of lotus seedpods obtained by non-organic and organic solvents exerted antioxidation, anti-cancer, anti-melanogenic, anti-inflammatory, anti-irradiation, cardioprotection and hepatoprotection activities. Meanwhile, extraction solvents and methods have influences on the activities. Diversity phytochemicals are responsible for these bioactivities, such as proanthocyanidins, oligomeric procyanidins, flavonoids, alkaloids, terpenoids, organic acids, steroids, esters and others. Some of phytochemicals have been well-identified by modern analytical techniques, and chemical structures of some phytochemicals have been proposed. Moreover, chemical assays as well as cell and animal experiments have demonstrated that phytochemicals (especially proanthocyanidins and oligomeric procyanidins) from lotus seedpods exhibited broad-spectrum biological activities, including ameliorating cognitive impairment, antioxidation, antibacterial, anti-glycative, neuroprotection, anti-tyrosinase, retinal protection, anti-insomnia, anti-cancer, immunomodulatory, ameliorating intestinal injury, anti-diabetes, regulating lipid homeostasis, anti-inflammatory and α-glucosidase inhibitory effects. Furthermore, raw material, extracts and phytochemicals of lotus seedpods have been applied as sources for biochar and biomass material, in food industry, and as dye. In contrast, other parts of lotus like seeds, stems, leaves, flowers, epicarps, plumules, stamens, petals and rhizomes have been reported to contain phytochemicals included flavonoids, glycosides, alkaloids, monosaccharides, essential oils, organic acids, chlorophylls, sesquiterpenoids, steroids, sapogenins, etc (2). Biological activities of these parts of lotus have been summarized as anti-obesity, antioxidant, cardiovascular, hepatoprotective, hypoglycemic, antimicrobial, lipolytic, anti-amnesic, anti-inflammatory, antithrombotic, anti-proliferative, memory-improving, sedative, immunoregulatory and antiviral activities (2). These parts of lotus can be applied in health food industry and medicine (2). Therefore, to lotus industry, lotus seedpods are worth exploiting for acquisition of novel phytochemicals, enrichment of more biological activities and new attempts of industrial applications.
However, there are some issues should be resolved in future: (1) the phytochemicals from lotus seedpod is mainly prepared using conventional solvent method, more attempts should be made to novel means; (2) although many phytochemicals with small molecular weight have been isolated and identified from lotus seedpods, the exploitations of those (like proteins and polysaccharides) with large molecular weight have been rarely done; (3) there is little information on digestion, absorption, distribution, structure-activity relationship, metabolism of phytochemicals from lotus seedpod, which need to be disclose with more work; (4) the current researches on biological activities of phytochemicals from lotus seedpods mostly foucused on proanthocyanidins and oligomeric procyanidin, the biological activities of other phytochemicals from lotus seedpod; (5) biological activities of extracts and phytochemicals from lotus seedpods have been indicated in chemical assays as well as cell and animal experiments, but have not been demonstrated by clinical research; (6) the action mechanisms about the relevant activities of extracts and phytochemicals from lotus seedpods are unclear as they have been preliminarily explored.
Author contributions
Y-FW: conceptualization, investigation, visualization, and writing—original draft. Z-CS and JL: investigation and visualization. TL, J-LS, and X-FL: investigation. Y-PL: project administration and funding acquisition. WZ: writing—review and editing. QZ: funding acquisition. X-YW: project administration, writing—review and editing, supervision, and funding acquisition. All authors contributed to the article and approved the submitted version.
Funding
This work was gratefully supported by the Science and Technology Research Project of Jiangxi Provincial Education Department (GJJ190805 and GJJ211507); the Open Project of Key Laboratory of Prevention and Treatment of Cardiovascular and Cerebrovascular Diseases, Ministry of Education (XN202001); University-Level Scientific Research Projects of Gannan Medical University (QD201913 and QD202128); and the Jiangxi Provincial College Students Innovation and Entrepreneurship Training Programs (S202210413028).
Conflict of interest
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Publisher’s note
All claims expressed in this article are solely those of the authors and do not necessarily represent those of their affiliated organizations, or those of the publisher, the editors and the reviewers. Any product that may be evaluated in this article, or claim that may be made by its manufacturer, is not guaranteed or endorsed by the publisher.
Supplementary material
The Supplementary Material for this article can be found online at: https://www.frontiersin.org/articles/10.3389/fnut.2022.1022794/full#supplementary-material
Abbreviations
DPPH, 1,1-diphenyl-2-picrylhydrazyl; ABTS, 2,2′-azino-bis-(3-ethylbenzthiazoline-6-sulfonic acid); OH, hydroxyl radical; O2–, superoxide anion; MDA, malondialdehyde; TBARS, thiobarbituric acid reactive substances; SOD, superoxide dismutase; CAT, catalase; GPX, glutathione peroxidase; GSH, glutathione; LPO, lipid peroxidation; ROS, reactive oxygen species; NO, nitric oxide; Ang II, angiotensin II; PKC, protein kinase C; p-PKC, phosphorylation PKC; ERK, extracellular-signal-regulated kinase; p-ERK, phosphorylation ERK; NADPH, nicotinamide adenine dinucleotide phosphate; NOX, NADPH oxidase; AT1R, type I angiotensin receptor; RAGE, receptor for advanced glycation end products; MAPK, mitogen-activated protein kinase; TRP, tyrosinase-related protein 1; PKA, protein kinase A; CREB, cAMP-response element binding protein; p-CREB, phosphorylation CREB; MITF, melanocyte inducing transcription factor; JNK, c-Jun N terminal kinase; p-JNK, phosphorylation JNK; AchE, acetylcholinesterase; MC1R, melanocortin-1 receptor; PARP, poly adenosine diphosphoribose polymerase; Axl, tyrosine kinase receptor; IL-1β, interleukin-1β; IL-2, interleukin-2; IL-6, interleukin-6; IL-8, interleukin-8; IL-10, interleukin-10; NF-κB, nuclear factor kappa B; p-NF-κB, phosphorylation NF-κB; Nrf2, nuclear respiratory factor 2; HO-1, heme oxygenase-1; LPS, lipopolysaccharide; TNF-α, tumor necrosis factor-α; COX-2, cyclooxygenase-2; NOS, nitric oxide synthase; iNOS, inducible NOS; nNOS, neuronal NOS; tNOS, total NOS; TLR4, toll-like receptor 4; MyD88, myeloid differentiation primary response gene 88; ZO-1, zonula occludens-1; CD14, cluster of differential 14; AGEs, advanced glycation end products; MAO-B, monoamine oxidase B; Aβ, β-Amyloid peptide; CaMKII, calcium/calmodulin-dependent protein kinase II; BDNF, brain-derived neurotrophic factor; DAG, diacylglycerol; IP3, ins(1,4,5)P3; PP2B, protein phosphatase 2B; T-AOC, total antioxidant capacity; ADMA, asymmetric dimethylarginine; DDAH, dimethylarginine dimethylaminohydrolase; 5-HT, 5-hydroxytryptamine; GABA, γ-aminobutyric acid; Glu, glutamic acid; NE, noradrenaline; mTOR, mammalian target of rapamycin; GK, glucokinase; FoxO1a, forkhead box O1; p-FoxO1a, phosphorylation FoxO1a; SREBP-1c, sterol regulatory element-binding protein 1c; G-6-Pase, glucose-6-phosphatase; ACC1, acetyl coenzyme A carboxylase 1; ACL, ATP-citrate lyase; FAS, fatty acid synthase; SCD1, stearoyl CoA desaturase 1; S6K1, S6 Kinase 1; PEPCK, phosphoenolpyruvate carboxykinase; PFK, phosphofructokinase; PK, pyruvate kinase; HK II, hexokinase II; UCP-1, uncoupling protein 1; GR, glutathione reductase; GST, glutathione-S-transferase; LDH, lactate dehydrogenase; AKT, protein kinase B; p-AKT, phosphorylation AKT; Ach, acetylcholine; NR2B, NMDA receptor 2B; ASK, apoptosis signal regulating kinase; CML, Nϵ-(carboxymethyl) lysine; FFA, free fatty acids; TC, cholesterol; TG, triglyceride; AST, aspartate aminotransferase; ALT, alanine aminotransferase; ALP, alkaline phosphatase; HDL-C, high-density lipoprotein cholesterol; GRAC, glutathione reductase; ICAM-1, intercellular adhesion molecule-1; LDL, low-density lipoprotein; VCAM-1, vascular cell adhesion molecule-1; LC3, light chain 3; Atg5/12, autophagy elongation complex; PI3K, phosphoinositide 3-kinase; Beclin-1, the mammalian ortholog of yeast ATG6; Bcl-2, B-cell lymphoma-2; Bax, Bcl2-associated X; Bad, Bcl2 associated death promoter; p-Bad, phosphorylation Bad; IκB, inhibitor of NF-κB; IKK, IκB kinase; p-IκB, phosphorylation IκB; γ-H2AX, phosphorylated histone family 2A variant; NFATc-1, nuclear factor of activated T cells cytoplasmic 1; ANP, atrial natriuretic peptide; BNP, B-type natriuretic peptide; MLC2, myosin light chain 2; ET-1, endothelin-1; SYN, synaptophysin; Arc, activity-regulated cytoskeleton−associated protein; INF-γ, gamma-interferon; GLUT, glucose transporter; PPAR, peroxisome proliferator-activated receptors; SULT2B1b, sulfotransferase 2B1b.
References
1. Zhang Y, Lu X, Zeng SX, Huang XH, Guo ZB, Zheng YF, et al. Nutritional composition, physiological functions and processing of lotus (Nelumbo nucifera Gaertn.) seeds: a review. Phytochem Rev. (2015) 14:321–34. doi: 10.1007/s11101-015-9401-9
2. Chen GL, Zhu MZ, Guo MQ. Research advances in traditional and modern use of Nelumbo nucifera: phytochemicals, health promoting activities and beyond. Crit Rev Food Sci. (2019) 59:S189–209. doi: 10.1080/10408398.2018.1553846
3. Limwachiranon J, Huang H, Shi ZH, Li L, Luo ZS. Lotus flavonoids and phenolic acids: health promotion and safe consumption dosages. Compr Rev Food Sci Food Saf. (2018) 17:458–71. doi: 10.1111/1541-4337.12333
4. Wu HW, Shu LP, Liang T, Li YP, Liu YX, Zhong XL, et al. Extraction optimization, physicochemical property, antioxidant activity, and α−glucosidase inhibitory effect of polysaccharides from lotus seedpods. J Sci Food Agr. (2022) 102:4065–78. doi: 10.1002/jsfa.11755
5. Ling ZQ, Xie BJ, Yang EL. Isolation, characterization, and determination of antioxidative activity of oligomeric procyanidins from the seedpod of Nelumbo nucifera Gaertn. J Agr Food Chem. (2005) 53:2441–5. doi: 10.1021/jf040325p
6. Wang JM, Yu T, Sheng LQ, Zhang H, Chen F, Zhu J, et al. Lotus seedpod proanthocyanidins protect against light-induced retinal damage via antioxidative stress, anti-apoptosis, and neuroprotective effects. Med Sci Monitor. (2021) 27:e935000. doi: 10.12659/MSM.935000
7. Wu Q, Feng YN, Ouyang Y, Liang YG, Zhao KQ, Wang Y, et al. Inhibition of advanced glycation endproducts formation by lotus seedpod oligomeric procyanidins through RAGE-MAPK signaling and NF-κB activation in high-AGEs-diet mice. Food Chem Toxicol. (2021) 156:112481. doi: 10.1016/j.fct.2021.112481
8. Shen YB, Guan YF, Song X, He JL, Xie ZX, Zhang YW, et al. Polyphenols extract from lotus seedpod (Nelumbo nucifera Gaertn.): phenolic compositions, antioxidant, and antiproliferative activities. Nutr Food sci. (2019) 7:3062–70. doi: 10.1002/fsn3.1165
9. Lee MS, Chyau CC, Wang CP, Wang TH, Chen JH, Lin HH. Flavonoids identification and pancreatic beta-cell protective effect of lotus seedpod. Antioxid Basel. (2020) 9:658. doi: 10.3390/antiox9080658
10. Huang H, Yan PP, Sun TP, Mo XX, Yin JW, Li PY, et al. Procyanidins extracted from lotus seedpod ameliorate amyloid-β-induced toxicity in rat pheochromocytoma cells. Oxid Med Cell Longev. (2018) 2018:4572893. doi: 10.1155/2018/4572893
11. Zhang HH, Dai YY, Cheng YX, He YQ, Manyakara Z, Duan YQ, et al. Influence of extremely low frequency magnetic fields on Ca2+ signaling and double messenger system in mice hippocampus and reversal function of procyanidins extracted from lotus seedpod. Bioelectromagnetics. (2017) 38:436–46. doi: 10.1002/bem.22058
12. Gong YS, Guo J, Hu K, Gao YQ, Xie BJ, Sun ZD, et al. Ameliorative effect of lotus seedpod proanthocyanidins on cognitive impairment and brain aging induced by D-galactose. Exp Gerontol. (2016) 74:21–8. doi: 10.1016/j.exger.2015.11.020
13. Bao NN, Rashed MM, Jiang BL, Zhai KF, Luo ZS. Green and efficient extraction approach for polyphenol recovery from lotus seedpods (receptaculum nelumbinis): gas-assisted combined with glycerol. ACS omega. (2021) 6:26722–31. doi: 10.1021/acsomega.1c04190
14. Yan Z, Zhang HH, Dzah CS, Zhang JX, Diao CR, Ma HL, et al. Subcritical water extraction, identification, antioxidant and antiproliferative activity of polyphenols from lotus seedpod. Sep Purif Technol. (2020) 236:116217. doi: 10.1016/j.seppur.2019.116217
15. Wang JY, Xie BJ, Sun ZD. The improvement of carboxymethyl β-glucan on the antibacterial activity and intestinal flora regulation ability of lotus seedpod procyanidins. LWT. (2021) 137:110441. doi: 10.1016/j.lwt.2020.110441
16. Wang JY, Bie M, Zhou WJ, Xie BJ, Sun ZD. Interaction between carboxymethyl pachyman and lotus seedpod oligomeric procyanidins with superior synergistic antibacterial activity. Carbohyd Polym. (2019) 212:11–20. doi: 10.1016/j.carbpol.2019.02.030
17. Wang JY, Zhang WJ, Tang CE, Xiao J, Xie BJ, Sun ZD. Synergistic effect of B-type oligomeric procyanidins from lotus seedpod in combination with water-soluble Poria cocos polysaccharides against E. coli and mechanism. J Funct Foods. (2018) 48:134–43. doi: 10.1016/j.jff.2018.07.015
18. Wu Q, Li SY, Li XP, Sui Y, Yang Y, Dong LH, et al. Inhibition of advanced glycation endproduct formation by lotus seedpod oligomeric procyanidins through RAGE–MAPK signaling and NF-κB activation in high-fat-diet rats. J Agr Food Chem. (2015) 63:6989–98. doi: 10.1021/acs.jafc.5b01082
19. Wu Q, Zhao KQ, Chen YY, Ouyang Y, Feng YN, Li S, et al. Effect of lotus seedpod oligomeric procyanidins on AGEs formation in simulated gastrointestinal tract and cytotoxicity in Caco-2 cells. Food Funct. (2021) 12:3527–38. doi: 10.1039/d0fo03152f
20. Li XP, Sui Y, Wu Q, Xie BJ, Sun ZD. Attenuated mTOR signaling and enhanced glucose homeostasis by dietary supplementation with lotus seedpod oligomeric procyanidins in streptozotocin (STZ)-induced diabetic mice. J. Agric. Food Chem. (2017) 65:3801–10. doi: 10.1021/acs.jafc.7b00233
21. Liu N, Liu YG, Zeng GM, Gong JL, Tan XF, Liu SB, et al. Adsorption of 17β-estradiol from aqueous solution by raw and direct/pre/post-KOH treated lotus seedpod biochar. J Environ Sci. (2020) 87:10–23. doi: 10.1016/j.jes.2019.05.026
22. Wei HL, Wang XN, Zhang DM, Du W, Sun XQ, Jiang FY, et al. Facile synthesis of lotus seedpod-based 3D hollow porous activated carbon/manganese dioxide composite for supercapacitor electrode. J Electroanal Chem. (2019) 853:113561. doi: 10.1016/j.jelechem.2019.113561
23. Feng NJ, She SW, Hu HF, Tang SM, Tan JY, Wu Q, et al. Effects of oligomeric procyanidins from lotus seedpod on the retrogradation properties of rice starch. Front Nutr. (2021) 8:751627. doi: 10.3389/fnut.2021.751627
24. Bangar SP, Dunno K, Kumar M, Mostafa H, Maqsood S. A comprehensive review on lotus seeds (Nelumbo nucifera Gaertn.): nutritional composition, health-related bioactive properties, and industrial applications. J Funct Foods. (2022) 89:104937. doi: 10.1016/j.jff.2022.104937
25. Wang ZY, Cheng Y, Zeng MM, Wang ZJ, Qin F, Wang YZ, et al. Lotus (Nelumbo nucifera Gaertn.) leaf: a narrative review of its phytoconstituents, health benefits and food industry applications. Trends Food Sci Tech. (2021) 112:631–50. doi: 10.1016/j.tifs.2021.04.033
26. Liu YT, Lai YH, Lin HH, Chen JH. Lotus seedpod extracts reduced lipid accumulation and lipotoxicity in hepatocytes. Nutrients. (2019) 11:2895. doi: 10.3390/nu11122895
27. Hsu JY, Lin HH, Li TS, Tseng CY, Wong YC, Chen JH. Anti-melanogenesis effects of lotus seedpod in vitro and in vivo. Nutrients. (2020) 12:3535. doi: 10.3390/nu12113535
28. Tseng HC, Tsai PM, Chou YH, Lee YC, Lin HH, Chen JH. In vitro and in vivo protective effects of flavonoid-enriched lotus seedpod extract on lipopolysaccharide-induced hepatic inflammation. Am J Chinese Med. (2019) 47:153–76. doi: 10.1142/S0192415X19500083
29. Kim MJ, Shin HS. Antioxidative effect of lotus seed and seedpod extracts. Food Sci Biotechnol. (2012) 21:1761–6. doi: 10.1007/s10068-012-0234-7
30. Cho S, Cho HW, Woo KW, Jeong J, Lim J, Park S, et al. Nelumbo nucifera receptaculum extract suppresses angiotensin II-induced cardiomyocyte hypertrophy. Molecules. (2019) 24:1647. doi: 10.3390/molecules24091647
31. Lee EJ, Seo YM, Kim YH, Chung C, Sung HJ, Sohn HY, et al. Anti-inflammatory activities of ethanol extracts from leaf, seed, and seedpod of Nelumbo nucifera. J Life Sci. (2019) 29:436–41. doi: 10.5352/JLS.2019.29.4.436
32. Kim NY, Yang IJ, Kim S, Lee C. Lotus (Nelumbo nucifera) seedpod extract inhibits cell proliferation and induces apoptosis in non-small cell lung cancer cells via downregulation of Axl. J. Food Biochem. (2021) 45:e13601. doi: 10.1111/jfbc.13601
33. Shin HJ, Kim M, Shin BS, Bae S, Shin HJ, Kim M, et al. Anti-melanogenic effect of lotus seed and seedpod extracts via downregulation of tyrosinase stability in B16F10 murine melanoma cells. Asian J Beauty Cosmetol. (2022) 20:111–20.
34. Hu WC, Wang GC, Shen T, Wang YN, Hu BR, Wang XF, et al. Chemical composition, antioxidant and cytoprotective activities of lotus receptacle. Hortic Environ Biotechnol. (2015) 56:712–20. doi: 10.1007/s13580-015-0091-4
35. Wu YB, Zheng LJ, Wu JG, Chen TQ, Yi J, Wu JZ. Antioxidant activities of extract and fractions from receptaculum nelumbinis and related flavonol glycosides. Int J Mol Sci. (2012) 13:7163–73. doi: 10.3390/ijms13067163
36. Bao NN, Wang D, Fu XZ, Xie HJ, Gao GZ, Luo ZS. Green extraction of phenolic compounds from lotus seedpod (receptaculum nelumbinis) assisted by ultrasound coupled with glycerol. Foods. (2021) 10:239. doi: 10.3390/foods10020239
37. Duan YQ, Zhang HH, Xie BJ, Yan Y, Li J, Xu F, et al. Whole body radioprotective activity of an acetone–water extract from the seedpod of Nelumbo nucifera Gaertn. seedpod. Food Chem Toxicol. (2010) 48:3374–84. doi: 10.1016/j.fct.2010.09.008
38. Li XP, Chen Y, Li SY, Chen M, Xiao J, Xie BJ, et al. Oligomer procyanidins from lotus seedpod regulate lipid homeostasis partially by modifying fat emulsification and digestion. J Agr Food Chem. (2019) 67:4524–34. doi: 10.1021/acs.jafc.9b01469
39. Xiao JS, Xie BJ, Cao YP, Wu H, Sun ZD, Xiao D. Characterization of oligomeric procyanidins and identification of quercetin glucuronide from lotus (Nelumbo nucifera Gaertn.) seedpod. J Agr Food Chem. (2012) 60:2825–9. doi: 10.1021/jf205331e
40. Wu YB, Zheng LJ, Yi J, Wu JG, Chen TQ, Wu JZ. Quantitative and chemical fingerprint analysis for the quality evaluation of receptaculum nelumbinis by RP-HPLC coupled with hierarchical clustering analysis. Int J Mol Sci. (2013) 14:1999–2010. doi: 10.3390/ijms14011999
41. Pei HT, Su WY, Gui M, Dou MJ, Zhang YX, Wang CZ, et al. Comparative analysis of chemical constituents in different parts of lotus by UPLC and QToF-MS. Molecules. (2021) 26:1855. doi: 10.3390/molecules26071855
42. Wu Q, Li S, Fu X, Yang T, Chen H, Guan Y, et al. Spectroscopic studies on binding of lotus seedpod oligomeric procyanidins to bovine serum albumin. J Appl Spectrosc. (2014) 80:884–92. doi: 10.1007/s10812-014-9860-6
43. Wu Q, Chen HY, Lv ZJ, Li SY, Hu B, Guan YF, et al. Oligomeric procyanidins of lotus seedpod inhibits the formation of advanced glycation end-products by scavenging reactive carbonyls. Food Chem. (2013) 138:1493–502. doi: 10.1016/j.foodchem.2012.10.111
44. Wu Q, Li SY, Yang T, Xiao J, Chu QM, Li T, et al. Inhibitory effect of lotus seedpod oligomeric procyanidins on advanced glycation end product formation in a lactose-lysine model system. Electron J Biotechnol. (2015) 18:68–76. doi: 10.1016/j.ejbt.2014.10.005
45. Limwachiranon J, Huang H, Li L, Duan ZH, Luo ZS. Recovery of lotus (Nelumbo nucifera Gaertn.) seedpod flavonoids using polar macroporous resins: the updated understanding on adsorption/desorption mechanisms and the involved intermolecular attractions and bonding. Food Chem. (2019) 299:125108. doi: 10.1016/j.foodchem.2019.125108
46. Wu J, Xu JG, Fu JP, Xiong W, Zhang SW, Gu Z, et al. Characterization of tyrosinase inhibitors from white lotus receptacle. Chem Nat Compd. (2019) 55:929–31. doi: 10.1007/s10600-019-02849-7
47. Cho HW, Jung WS, An BG, Cho JH, Jung SY. Isolation of compounds having inhibitory activity toward tyrosinase from receptaculum nelumbinis. Korean J Pharmacogn. (2013) 44:1–5.
48. Zhang JX, Zhang XX, Wen CT, Duan YQ, Zhang HH. Lotus seedpod proanthocyanidins protect against neurotoxicity after methyl-mercuric chloride injury. Ecotox Environ Safe. (2019) 183:109560. doi: 10.1016/j.ecoenv.2019.109560
49. Wu Q, Ouyang Y, Feng YN, Kong YF, Liang YG, Zhang C, et al. Comparative study of the inhibitory effects of lotus seedpod oligomeric procyanidins on dietary AGE released from glycated casein during digestion. Food Res Int. (2022) 152:110912. doi: 10.1016/j.foodres.2021.110912
50. Liu HT, Liu JS, Zhang J, Qi YD, Jia XG, Zhang BG, et al. Simultaneous quantitative and chemical fingerprint analysis of receptaculum nelumbinis based on HPLC–DAD-MS combined with chemometrics. J Chromatogr Sci. (2016) 54:618–24. doi: 10.1093/chromsci/bmv229
51. Huang H, Belwal T, Jiang L, Hu JW, Limwachiranon J, Li L, et al. Valorization of lotus byproduct (receptaculum nelumbinis) under green extraction condition. Food Bioprod Process. (2019) 115:110–7. doi: 10.1016/j.fbp.2019.03.006
52. Xiao J, Sui Y, Li SY, Wu Q, Yang T, Xie BJ, et al. Combination of proanthocyanidins extracted from lotus seedpod and L-cysteine ameliorates memory impairment induced by alcohol and scopolamine in mice. Eur Food Res Technol. (2013) 236:671–9. doi: 10.1007/s00217-013-1922-0
53. Xiao J, Li SY, Sui Y, Wu Q, Li XP, Xie BJ, et al. Lactobacillus casei-01 facilitates the ameliorative effects of proanthocyanidins extracted from lotus seedpod on learning and memory impairment in scopolamine-induced amnesia mice. PLoS One. (2014) 9:e112773. doi: 10.1371/journal.pone.0112773
54. Xiao J, Li SY, Sui Y, Li XP, Wu Q, Zhang RF, et al. In vitro antioxidant activities of proanthocyanidins extracted from the lotus seedpod and ameliorative effects on learning and memory impairment in scopolamine-induced amnesia mice. Food Sci Biotechnol. (2015) 24:1487–94.
55. Xu JQ, Rong S, Xie BJ, Sun ZD, Zhang L, Wu HL, et al. Procyanidins extracted from the lotus seedpod ameliorate scopolamine-induced memory impairment in mice. Phytother Res. (2009) 23:1742–7. doi: 10.1002/ptr.2837
56. Duan YQ, Wang ZG, Zhang HH, He YQ, Lu RZ, Zhang R, et al. The preventive effect of lotus seedpod procyanidins on cognitive impairment and oxidative damage induced by extremely low frequency electromagnetic field exposure. Food Funct. (2013) 4:1252–62. doi: 10.1039/c3fo60116a
57. Duan YQ, Wang ZG, Zhang HH, He YQ, Fan R, Cheng YX, et al. Extremely low frequency electromagnetic field exposure causes cognitive impairment associated with alteration of the glutamate level, MAPK pathway activation and decreased CREB phosphorylation in mice hippocampus: reversal by procyanidins extracted from the lotus seedpod. Food Funct. (2014) 5:2289–97. doi: 10.1039/c4fo00250d
58. Xu JQ, Rong S, Xie BJ, Sun ZD, Zhang L, Wu HL, et al. Rejuvenation of antioxidant and cholinergic systems contributes to the effect of procyanidins extracted from the lotus seedpod ameliorating memory impairment in cognitively impaired aged rats. Eur. Neuropsychopharmacol. (2009) 19:851–60. doi: 10.1016/j.euroneuro.2009.07.006
59. Xu JQ, Rong S, Xie BJ, Sun ZD, Deng QC, Wu HL, et al. Memory impairment in cognitively impaired aged rats associated with decreased hippocampal CREB phosphorylation: reversal by procyanidins extracted from the lotus seedpod. J Gerontol A Biol. (2010) 65:933–40. doi: 10.1093/gerona/glq094
60. Xu JQ, Rong S, Xie BJ, Sun ZD, Deng QC, Bao W, et al. Changes in the nitric oxide system contribute to effect of procyanidins extracted from the lotus seedpod ameliorating memory impairment in cognitively impaired aged rats. Rejuv Res. (2011) 14:33–43. doi: 10.1089/rej.2010.1076
61. Gong YS, Liu LG, Xie BJ, Liao YC, Yang EL, Sun ZD. Ameliorative effects of lotus seedpod proanthocyanidins on cognitive deficits and oxidative damage in senescence-accelerated mice. Behav Brain Res. (2008) 194:100–7. doi: 10.1016/j.bbr.2008.06.029
62. Qin Y, Sun Y, Li JQ, Xie RP, Deng ZY, Chen HB, et al. Characterization and antioxidant activities of procyanidins from lotus seedpod, mangosteen pericarp, and camellia flower. Int J Food Prop. (2017) 20:1621–32. doi: 10.1080/10942912.2016.1215997
63. Luo XP, Chen M, Duan YQ, Duan WY, Zhang HH, He YQ, et al. Chemoprotective action of lotus seedpod procyanidins on oxidative stress in mice induced by extremely low-frequency electromagnetic field exposure. Biomed Pharmacother. (2016) 82:640–8. doi: 10.1016/j.biopha.2016.06.005
64. Xu JQ, Rong S, Xie BJ, Sun ZD, Zhang L, Wu HL, et al. Procyanidins extracted from the lotus seedpod ameliorate age-related antioxidant deficit in aged rats. J Gerontol A Biol. (2010) 65:236–41. doi: 10.1093/gerona/glp211
65. Tang CE, Xie BJ, Sun ZD. Antibacterial activity and mechanism of B-type oligomeric procyanidins from lotus seedpod on enterotoxigenic Escherichia coli. J Funct Foods. (2017) 38:454–63. doi: 10.1016/j.jff.2017.09.04
66. Yin CC, Luo XP, Duan YQ, Duan WY, Zhang HH, He YQ, et al. Neuroprotective effects of lotus seedpod procyanidins on extremely low frequency electromagnetic field-induced neurotoxicity in primary cultured hippocampal neurons. Biomed Pharmacother. (2016) 82:628–39. doi: 10.1016/j.biopha.2016.05.032
67. Xiao HB, Wang YS, Liang L, Lu XY, Sun ZL. Procyanidin B2 from lotus seedpod regulate NO/ADMA/DDAH pathway to treat insomnia in rats. Fund Clin Pharmacol. (2019) 33:549–57. doi: 10.1111/fcp.12462
68. Duan YQ, Xu H, Luo XP, Zhang HH, He YQ, Sun GB, et al. Procyanidins from Nelumbo nucifera Gaertn. Seedpod induce autophagy mediated by reactive oxygen species generation in human hepatoma G2 cells. Biomed Pharmacother. (2016) 79:135–52. doi: 10.1016/j.biopha.2016.01.039
69. Zhang HH, Cheng YX, Luo XP, Duan YQ. Protective effect of procyanidins extracted from the lotus seedpod on immune function injury induced by extremely low frequency electromagnetic field. Biomed Pharmacother. (2016) 82:364–72. doi: 10.1016/j.biopha.2016.05.021
70. Tang CE, Xie BJ, Zong Q, Sun ZD. Proanthocyanidins and probiotics combination supplementation ameliorated intestinal injury in enterotoxigenic Escherichia coli infected diarrhea mice. J Funct Foods. (2019) 62:103521. doi: 10.1016/j.jff.2019.103521
71. Xie CQ, Wang SF, Cao MY, Xiong W, Wu L. (E)-9-octadecenoic acid ethyl ester derived from lotus seedpod ameliorates inflammatory responses by regulating MAPKs and NF-κB signalling pathways in LPS-Induced RAW264. 7 macrophages. Evid Based Compl Altern Med. (2022) 2022:6731360. doi: 10.1155/2022/6731360
72. Wu Q, Li SY, Li XP, Fu XY, Sui Y, Guo TT, et al. A significant inhibitory effect on advanced glycation end product formation by catechin as the major metabolite of lotus seedpod oligomeric procyanidins. Nutrients. (2014) 6:3230–44. doi: 10.3390/nu6083230
73. Chen Z, Liu T, Tang JJ, Zheng ZJ, Wang HM, Shao Q, et al. Characteristics and mechanisms of cadmium adsorption from aqueous solution using lotus seedpod-derived biochar at two pyrolytic temperatures. Environ Sci Pollut Res Int. (2018) 25:11854–66. doi: 10.1007/s11356-018-1460-1
74. Rao LM, Zhu YF, Duan ZS, Xue T, Duan XM, Wen YP, et al. Lotus seedpods biochar decorated molybdenum disulfide for portable, flexible, outdoor and inexpensive sensing of hyperin. Chemosphere. (2022) 301:134595. doi: 10.1016/j.chemosphere.2022.134595
75. Qin YT, Ni C, Xie XB, Zhang JJ, Wang BL, Wu HT, et al. Multiple reflection and scattering effects of the lotus seedpod-based activated carbon decorated with Co3O4 microwave absorbent. J Colloid Interf Sci. (2021) 602:344–54. doi: 10.1016/j.jcis.2021.06.048
76. Xie ZL, Sun JF, Zhou ZJ, Xia SB. Lotus seedpod-based carbon quantum dots: preparation, characterization and application For Fe (III) Detection. Mater Technol. (2021) 55:141–7. doi: 10.17222/mit.2020.160
77. Zheng B, Wang JX, Pan ZR, Wang XF, Liu SX, Ding SQ, et al. An efficient metal-free catalyst derived from waste lotus seedpod for oxygen reduction reaction. J Porous Mat. (2020) 27:637–46. doi: 10.1007/s10934-019-00846-3
78. Pu J, Kong W, Lu CC, Wang ZH. Directly carbonized lotus seedpod shells as high-stable electrode material for supercapacitors. Ionics. (2015) 21:809–16. doi: 10.1007/s11581-014-1225-x
79. Wu F, Zhang MH, Bai Y, Wang XR, Dong RQ, Wu C. Lotus seedpod-derived hard carbon with hierarchical porous structure as stable anode for sodium-ion batteries. ACS Appl Mater Inter. (2019) 11:12554–61. doi: 10.1021/acsami.9b01419
80. Hu L, Liu S, Pan YT, Huang LY, Cui QJ, Huang YJ, et al. Preparation of super activated carbon from various parts of Nelumbo nucifera and its application as electrode material in supercapacitors. Int J Electrochem Sci. (2021) 16:21065. doi: 10.20964/2021.06.48
81. He QL, Wang HY, Zhang J, Zou ZC, Zhou J, Yang K, et al. Lotus seedpod as a low-cost biomass for potential methylene blue adsorption. Water Sci Technol. (2016) 74:2560–8. doi: 10.2166/wst.2016.423
82. Lee S, Shin HS. Effect of lotus seed and seedpod extracts on oxidative stability against lard during storage. J Korean Soc Appl Biol Chem. (2015) 58:53–60. doi: 10.1007/s13765-015-0006-1
83. Chen YS, Huang FH, Xie BJ, Sun ZD, McClements DJ, Deng QC. Fabrication and characterization of whey protein isolates-lotus seedpod proanthocyanin conjugate: its potential application in oxidizable emulsions. Food Chem. (2021) 346:128680. doi: 10.1016/j.foodchem.2020.128680
84. Chen YS, Zhang RJ, Xie BJ, Sun ZD, McClements DJ. Lotus seedpod proanthocyanidin-whey protein complexes: impact on physical and chemical stability of β-carotene-nanoemulsions. Food Res Int. (2020) 127:108738. doi: 10.1016/j.foodres.2019.108738
85. Chen YS, Huang FH, McClements DJ, Xie BJ, Sun ZD, Deng QC. Oligomeric procyanidin nanoliposomes prevent melanogenesis and UV radiation-induced skin epithelial cell (HFF-1) damage. Molecules. (2020) 25:1458. doi: 10.3390/molecules25061458
86. Feng NJ, Shen Y, Hu CQ, Tan JY, Huang Z, Wang C, et al. Inhibition of advanced glycation end products in yogurt by lotus seedpod oligomeric procyanidin. Front Nutr. (2021) 8:781998. doi: 10.3389/fnut.2021.781998
Keywords: lotus seedpod, phytochemicals, health benefits, potential application, food by-product
Citation: Wang Y-F, Shen Z-C, Li J, Liang T, Lin X-F, Li Y-P, Zeng W, Zou Q, Shen J-L and Wang X-Y (2022) Phytochemicals, biological activity, and industrial application of lotus seedpod (Receptaculum Nelumbinis): A review. Front. Nutr. 9:1022794. doi: 10.3389/fnut.2022.1022794
Received: 19 August 2022; Accepted: 12 September 2022;
Published: 04 October 2022.
Edited by:
Wei Liu, Nanchang University, ChinaReviewed by:
Chaoyang Ma, Jiangnan University, ChinaZhenxing Wang, Southwest Forestry University, China
Copyright © 2022 Wang, Shen, Li, Liang, Lin, Li, Zeng, Zou, Shen and Wang. This is an open-access article distributed under the terms of the Creative Commons Attribution License (CC BY). The use, distribution or reproduction in other forums is permitted, provided the original author(s) and the copyright owner(s) are credited and that the original publication in this journal is cited, in accordance with accepted academic practice. No use, distribution or reproduction is permitted which does not comply with these terms.
*Correspondence: Xiao-Yin Wang, xywang@gmu.edu.cn
 Yi-Fei Wang1
Yi-Fei Wang1  Qi Zou
Qi Zou Xiao-Yin Wang
Xiao-Yin Wang