The tail-tale of stress: an exploratory analysis of cortisol levels in the tail-hair of captive Asian elephants
- Published
- Accepted
- Received
- Academic Editor
- Matt Sponheimer
- Subject Areas
- Animal Behavior, Conservation Biology, Ecology, Zoology, Anatomy and Physiology
- Keywords
- Hair cortisol, Asian elephant, Elephas maximus, Tail hair, Stress, Stress physiology
- Copyright
- © 2021 Pokharel et al.
- Licence
- This is an open access article distributed under the terms of the Creative Commons Attribution License, which permits using, remixing, and building upon the work non-commercially, as long as it is properly attributed. For attribution, the original author(s), title, publication source (PeerJ) and either DOI or URL of the article must be cited.
- Cite this article
- 2021. The tail-tale of stress: an exploratory analysis of cortisol levels in the tail-hair of captive Asian elephants. PeerJ 9:e10445 https://doi.org/10.7717/peerj.10445
Abstract
Background
Assessment of physiological states by measuring biomarkers, such as cortisol, has significantly contributed to the monitoring of health, welfare and management of animals. Immunoreactive cortisol in hair (hC) has been used widely for deciphering ‘stressful’ past-events in various wild and captive animals. However, no such studies have been done in long-lived mammals.
Methods
In this first exploratory study in elephants, we assessed (i) tail-hair growth rate (TGR) and (ii) hC levels in tail-hair samples from six captive Asian elephants from two zoos in Japan for comparing hC levels with zoo-keepers’ records of distinct biological events over a c.0.5–2.0-year period. Tail-hair samples were cut into segments (based on monthly growth rate), pulverized or minced and a validated cortisol enzyme-immunoassay employed to measure hC levels.
Results
When the hC levels of all individuals were compared with the keepers’ records, a posteriori, most of the high hC levels were found to be associated with ‘stressful’ or distinct behavioural events such as pathological (anaemia, colic infection, skin infection, oral sores), psychosocial (reluctance in entering the enclosure, presence of a calf) and husbandry practice-related (contact trials/ space sharing) conditions, indicating that tail-hair indeed can be a potential ‘retrospective’ calendar of physiological health of an animal.
Conclusions
Our observations open up the possibility of using the tail-hair as an alternative matrix to reconstruct the physiological history of elephants.
Introduction
Health status and well-being of both captive and wild animals are generally assessed by studying the behavioural, psychological and physiological changes, and their influences on animals’ reproduction and survival. One of the physiological changes, widely studied across taxa, is the assessment of the evolutionarily-conserved phenomenon, the stress response, mediated by the activation of hypothalamic-pituitary-adrenal (HPA) axis in response to various stressors and marked by the elevation of cortisol or corticosterone (Wingfield et al., 1998; Moberg, 2000; Möstl & Palme, 2002; Sheriff et al., 2011; MacDougall-Shackleton et al., 2019). Glucocorticoids are metabolic hormones having pleiotropic effects, primarily aiding in energy mobilization, and have been associated with stress response of an animal (Möstl & Palme, 2002; Sheriff et al., 2011; MacDougall-Shackleton et al., 2019). Out of multiple metabolic functions, glucocorticoids help an organism in coping with threats to its homeostasis; however, if such threats remain for a prolonged period, the accelerated rise in glucocorticoids has adverse effects on the overall health of an organism (Moberg, 2000; Möstl & Palme, 2002; Sheriff et al., 2011; Romero & Wingfield, 2016). Thus, these metabolic hormones are broadly referred to as ‘stress’ hormones (Möstl & Palme, 2002).
There are several invasive, minimally invasive and non-invasive indices used for assessing the levels of glucocorticoids in biological samples such as plasma, urine, faeces, hair, scute and baleen (Sheriff et al., 2011; Hamilton et al., 2018; Trumble et al., 2018; Hunt et al., 2018; Palme, 2019). Each of these indices has its own merits and demerits (Sheriff et al., 2011). Welfare concerns have spurred interest in examining the consequences of long-term ‘stress’ in captive and free-ranging animals, through reconstruction of the stress history of individuals. In this context, the technique developed for determining levels of cortisol in animal hair is gaining popularity (Russell et al., 2012; Heimbürge, Kanitz & Otten, 2019). Hair cortisol (hC; also refers to immunoreactive cortisol) levels are considered effective for measuring the cumulative concentrations of systemic cortisol exposure over a longer period and have been extensively used across different taxa (Russell et al., 2012; Yamanashi, 2018; Heimbürge, Kanitz & Otten, 2019). Based on the multi-compartment model by Henderson (1993), cortisol is assumed to enter hair through passive diffusion from blood capillaries and, in addition, it is speculated that the local production of cortisol or contamination through sebaceous and sweat glands may contribute to hC levels (Keckeis et al., 2012; Russell et al., 2012; Salaberger et al., 2016; Heimbürge, Kanitz & Otten, 2019; Kalliokoski, Jellestad & Murison, 2019). However, the cortisol incorporation pathway in hair has not been studied and remains highly debatable (Russell et al., 2012; Kalliokoski, Jellestad & Murison, 2019). Nevertheless, cortisol levels in animal hair may still provide insights into the stress history of an individual animal.
Multiple studies have measured the hC levels in relation to various endogenous and exogenous factors (such as methodological validation, long-term stress, climatic variation, age-sex variation, reproductive status, dietary differences, social status and environment, habitat/landscape, anthropogenic factors) in several wild and captive species including rock hyrax (Procavia capensis: Koren et al., 2002; Koren, Mokady & Geffen, 2008), grizzly bear (Ursus arctos: Macbeth et al., 2010), polar bear (Ursus maritimus: Bechshøft et al., 2013), black bear (Ursus americanus: Lafferty et al., 2015), Siberian flying squirrel (Pteromys Volans: Santangeli et al., 2019), and many non-human primates (Carlitz et al., 2016; Yamanashi, 2018; Heimbürge, Kanitz & Otten, 2019). However, till date, no studies have been conducted either in Asian or African elephants to check the utility of hair cortisol.
The tail-hair of elephants, particularly, is distinct from hair in other large mammals in its thickness and also serves as a repository of chemical information (Cerling et al., 2006; Cerling et al., 2009; Wittemyer, Cerling & Douglas-Hamilton, 2009; Raha et al., 2013). A diverse range of biological questions from assessing protein structures to seasonal dietary patterns (Valente, 1983; Hillary & Buys, 1984; Cerling et al., 2006; Cerling et al., 2009) has been answered using elephant hair; yet, no attempts have been made so far to assess the physiological and reproductive biomarkers in body hair or tail-hair of elephants.
Physiological responses of both captive and wild Asian elephants (Elephas maximus) towards multiple ecological and anthropogenic stressors, such as seasonality, herd dynamics, habitat and dietary effects, housing, zoo environment and welfare outcomes, have been well-documented (Brown et al., 2019; Kumar et al., 2019; Palme, 2019; Pokharel et al., 2019; Pokharel, Seshagiri & Sukumar, 2017; Pokharel, Seshagiri & Sukumar, 2020). As elephants undergo several physiological and behavioural changes, individuals maintained under captive conditions require constant monitoring of their health parameters which might pose numerous challenges to keepers (Mason & Veasey, 2010; Brown et al., 2019). Therefore, several assays have been developed to facilitate evaluation of the health status and well-being of captive elephants. Mason & Veasey (2010) highlighted the potential significance of a retrospective investigation of cortisol using hair samples. In the context of wild elephants, the collection of hair samples may not be always feasible except perhaps during an autopsy of deceased elephants or when an animal is restrained for management or research purposes. Thus, the hC analysis may aid in reconstructing the long-term effects of ecological and anthropogenic stressors on wild populations.
This study investigated the potential utility of tail-hair as an alternative matrix to assess the physiological history by measuring the hC levels over time in growing hair. Being the first study in elephants, the objectives were designed to test the preliminary hypothesis based on the segmental deposition of cortisol within the hair shaft over a period of time, thereby acting as a ‘retrospective’ calendar of an animal’s physiological past (Russell et al., 2012). Thus, the main objectives of this study were to: (i) assess the growth rate of tail-hair, (ii) conduct a segment-wise analysis for obtaining time-series values of hC levels in the tail-hair of captive Asian elephants and (iii) to relate the obtained segment-wise hC levels to recorded behavioural or health-related events maintained by the elephant keepers, a posteriori.
Materials & Methods
All methods were performed in accordance with the World Association of Zoos and Aquariums Ethical Guidelines for the Conduct of Research on Animals by Zoos and Aquariums, and all experimental protocols were approved by the Animal Welfare and Animal Care Committee of the Wildlife Research Center (WRC-2018-004A) of Kyoto University, Japan. The collection of tail-hair samples from the elephants was permitted by the Directors, Hiroaki Katayama (Kyoto City Zoo) and Hiroyuki Ueyama (Kobe Oji Zoo), of the respective zoos.
Study animals and their history
Tail-hair samples were collected from six captive Asian elephants housed in the Kyoto City Zoo (KCZ) and Kobe Oji Zoo (KOZ), Japan; of these, two were adult females (Mito from the KCZ and Zuze from the KOZ), one was a sub-adult female, and three were juveniles (two females and one male from the KCZ). Most of the study animals experienced similar living conditions and adhered to similar dietary habits. The detailed history regarding the studied elephants (their age and years in captivity are reported till the study year 2018) is provided in Table 1 (Fig. S1). The daily records maintained by the keepers on behavioural and health status of these elephants were also accessed with the approval of the zoo authorities.
| S.No. | Study animal | Individual history | Average monthly tail-hair growth (mm) ±SD | Hair segments corresponding to previous months | Sample type | |||
|---|---|---|---|---|---|---|---|---|
| Age | Sex | Zoo | Years in captivity | |||||
| 1 | Mito | 48 | Female | KCZ | 39 years | 15.56 ± 4.19 | 22 | Powdered |
| 14 | Minced | |||||||
| 2 | Tonkun | 10 | Female | KCZ | 4 years | 10.83 ± 0.83 | 5 | Powdered |
| 3 | Bunnyun | 7 | Female | KCZ | 4 years | 13.61 ± 1.27 | 6 | Powdered |
| 4 | Kampart | 8 | Female | KCZ | 4 years | 12.78 ± 0.96 | 5 | Powdered |
| 5 | Tonkamu | 6 | Male | KCZ | 4 years | 20.00 ± 1.92 | 11 | Powdered |
| 6 | Zuze | 28 | Female | KOZ | 28 years | 19.40 ± 0.02 | 12 | Minced |
Tail-hair collections and growth rate
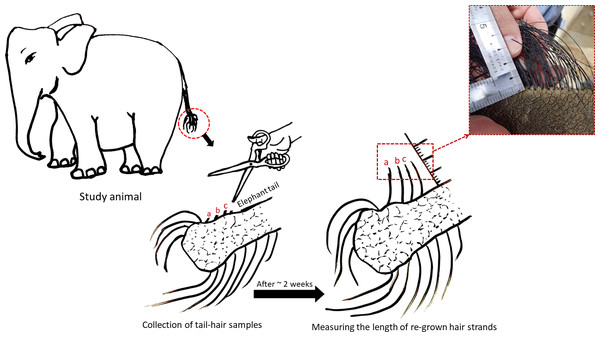
Elephant hair are most apparent around the eyes, ears, genital areas, chin and tail. Body hair in elephants are relatively thinner and sparsely present, but tail-hair are thicker, longer and easier to collect (Fowler & Mikota, 2006). Therefore, tail-hair samples were preferred over body hair and were collected by the elephant keepers in accordance with the recommendations in the “Guide for Animal Research Ethics” of the Wildlife Research Center, Kyoto University (WRC-2018-004A). Approvals from KCZ and KOZ authorities were taken to conduct this study. The individual tail-hair strand was cut as close as possible to the skin, avoiding any damage to the skin and to the tail-hair follicle. As elephants use their tail-hair for scratching the skin and swatting away insects, only a few strands (nearly 5 to 10 strands depending upon the thickness) were collected from an individual animal, labelled and were stored separately. The hair growth rate in Asian elephants had not been documented previously, therefore, an assessment of the growth rate of tail-hair in all study elephants was performed. To assess the growth rate, the remains of cut hair strands (nearly 3 strand remains to reduce handling-related disturbances) were alphabetically marked in the photograph and the growth of marked hair strands was measured after two to three weeks (based on the accessibility to the zoo facility: tail-hair growth was measured after 18 days for elephants in KCZ and after 14 days for Zuze in KOZ; see Fig. 1; Table S1). The monthly tail-hair growth rate for each individual was calculated by measuring the daily tail-hair growth (i.e., the total length of regrown hair divided by the number of days after sample collection; Fig. 1) and multiplying it by the total number of days in a month. To avoid any temporal influences on the hair growth, the tail-hair were collected within a month.
Figure 1: Assessing the daily growth rate of tail-hair.
The figure illustrates the assessment of the daily tail-hair growth rate by measuring the length of the re-grown hair after two weeks (18 days for the Kyoto City Zoo elephants and 14 days for Zuze in the Kobe Oji Zoo) of tail-hair collection. The daily tail-hair growth rate was extrapolated to calculate the monthly growth rate (refer to Table S1 for the results). Illustrated by Sanjeeta Sharma Pokharel.Tail-hair sample preparation
The length of each collected hair sample was measured before washing and decontamination. Since the tail-hair strands were thick (thickness ranging from 0.6 mm to 1.5 mm), they were initially wiped with 70% isopropanol (three times) to remove adhered dirt and faecal matter. These strands were then washed thrice with 70% isopropanol by rigorously shaking for 2 mins per wash. The washed samples were freeze-dried overnight to remove the moisture. The dried hair strands were then measured from tip to root of the strand. Each hair strand belonging to a single individual was cut from the proximal to the distal ends based on their monthly tail-hair growth rate (assuming that each segment corresponds with the previous month) and stored separately in labeled tubes for further analyses. The hair growth rate may vary between seasons, age-classes, sexes, dietary habits, reproductive states, etc (Randall & Ebling, 1991; Rushton, 2002; Randall, 2008; Bertoli et al., 2020); however, for the current study the monthly growth rate was assumed to be uniform across the tail-hair and segments were prepared accordingly.
Each hair segment, representing growth over 1-month, was then pulverized, using two-three beads (3.0 mm Zirconia beads, ZB-30, Tomy Seiko Co., LTD., Tokyo, Japan) and the crusher (MS-100, Tomy Seiko Co., LTD.), for 5 min (three times). Since some of the elephant tail-hair strands were quite thick (thickness ranging from 0.6 mm to 1.5 mm), they were further ground thrice or until the powder was obtained. To assess the influence of pulverization (grinding) and mincing, the tail-hair samples from Mito were manually minced. Samples were minced by finely cutting the monthly hair segments into pieces of roughly uniform size representing the daily hair growth rate (see Fig. S2). In the case of Zuze alone, samples were prepared only by mincing without pulverization. There were a total of 49 pulverized samples (corresponding to: 22 previous months (April 2018 to July 2016) for Mito, 5 previous months for Tonkun, 6 for Bunnyun, 5 for Kampart and 11 for Tonkamu) and 26 minced samples (corresponding to: 14 previous months (total segments were for 24 previous months, a few of them were combined due to low sample weight <0.05 gm) for Mito and 12 previous months (total hair segments were for 19 previous months, a few of the segments were combined due to low sample weight) for Zuze). hC levels obtained from minced hair segments that were pooled for more than 3 months were not used for comparisons.
Hair cortisol extraction and analyses
Hair cortisol was extracted and analyzed adopting the protocol defined in Yamanashi et al. (2013); Yamanashi et al. (2016) using 50 mg of pulverized and minced samples. The experiments pertaining to recovery rate and hC variations across body were not performed due to limited access to samples and sensitivity of elephants towards body hair, respectively. The collected tail-hair samples were extracted by adding one ml of 80% methanol to each weighed sample and incubating these samples for 24 h at 50 °C (with shaking at 700 rpm; Incubator SIC-320LW, AS ONE Co., Osaka, Japan). After incubation, extracted samples were centrifuged at 3,000 rpm for 2 mins at 25 °C and 600 µl supernatant was transferred into a 2 ml tube. The tubes with the supernatant were opened and kept for drying (to evaporate methanol) inside the box with silica gel which was placed over the hot plate at 50 °C. Once the sample vials were completely dried (∼20 to 24 h), these dried extracts were reconstituted with 200 µl of the EIA phosphate buffer, were vortexed for a minute and stored at −20 °C for further analyses.
The levels of tail-hair cortisol (hC) were analyzed using the cortisol enzyme-linked immunosorbent assay (EIA) with FKA404E antibody and FKA403 antigen (horseradish peroxidase conjugated cortisol; Cosmo Bio Co., Ltd.) based on Kinoshita et al. (2011). Sample extracts were diluted in the ratio of 1:2 and assessed in duplicate. Inter-assay and intra-assay coefficients of variation (CV) were measured to assess the plate-to-plate consistency and the variance between data points within an assay, respectively; they were found to be 10.23% and 3.77% for the cortisol assay using pulverized hair samples, and 10.78% and 3.42% while using the minced samples, respectively (see Fig. S3 for the standard curve and parallelism). The software Microplate Manager 6 (MPM) was used for calculating the cortisol concentrations from the obtained optical densities.
Data analyses
The current study being exploratory in nature, the primary focus was to assess the likelihood of obtaining retrospective patterns of hC levels in the tail-hair segments of individual elephants. This study, thus, aimed at comparing the hC levels within an individual and does not perform comparisons between elephants. Only descriptive statistics were computed to assess the individual-wise temporal patterns of tail hC levels for 6 captive individuals. After the completion of hC analyses in all samples, qualitative a posteriori comparisons were made to associate some of the hC levels obtained in an individual with distinct biological events recorded by the elephant keepers for that individual. For the convenience of comparisons of high and low values in an individual with these biological events, each recorded event was divided under five sub-categories depending upon the nature and type of stress. These categories consist of social (included: mainly interaction with other individuals), psychosocial (included: unexplained behavioural changes, sleep reluctance, behavioural changes during presence and transfer of a calf), reproductive (included: signs of a reproductively receptive period in female elephants or ‘estrus’ (Hess, Schmidt & Schmidt, 1983; Slade-Cain, Rasmussen & Schulte, 2008); sexual behaviours such as mounting attempts), pathological (included: infections, skin diseases, sores, abrasion) and husbandry practice-related (included: contact trials, interaction training) stressors. These factors have been documented to influence the cortisol levels in free-ranging elephants (in a faecal matrix: Ganswindt et al., 2010; Pokharel, Seshagiri & Sukumar, 2017; Pokharel, Seshagiri & Sukumar, 2020; Pokharel et al., 2019). Based on the hC levels of preceding months, values were considered to be either ‘high’ or ‘low’ for an individual. These changes in hC levels in each category were expressed either as percentage change (total increase/decrease in hC levels divided by hC levels of previous/ following months, multiplied by 100) or expressed as fold-change (the ratio of increase/decrease in hC levels over hC levels of previous/following months) when they exceeded by 100%. However, in the absence of a standard baseline cortisol level corresponding to a ‘no-stress scenario’, cut-off values for significant percentage (fold) changes were not suggested. Mean and standard deviation were reported as mean (sd). The difference in extraction methods, i.e. powdering and mincing (only for those months where hair segments were not combined) of Mito’s tail-hair segments, were compared using the Wilcoxon signed rank exact test (using the function wilcox.text) in the R software (R Core Team, 2020).
Results
Tail-hair growth rate
Tail-hair growth rate varied across individuals but not in the same individual (refer to standard deviation in Table 1). The daily growth rate of tail-hair ranged from 0.36 mm to 0.67 mm with the mean monthly tail-hair growth rate varying from 10.83 (0.83) mm to 20.00 (1.92) mm in a 10-year old female, Tonkun, and a 6-year old male, Tonkamu, respectively (refer to Table 1, Table S1, Supplementary Results). The longest hair length in individuals ranged between 5.7 cm (in Tonkun) and 40.0 cm (in Mito). Based on the growth rate and length of the tail-hair, the month-wise hair segments (corresponding to the retrospective months) represented the growth of 5 months to 22 months (Table 1).
Tail-hair cortisol in powdered versus minced samples
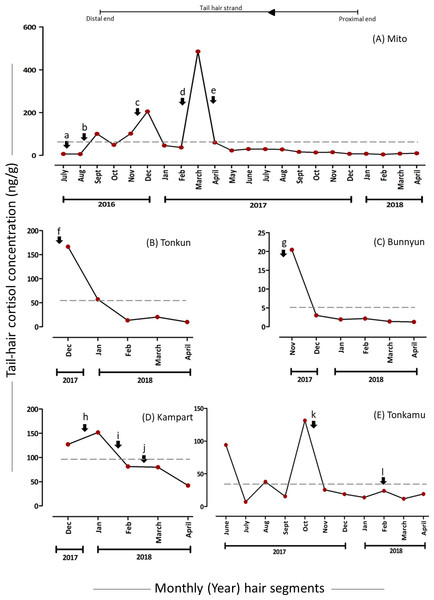
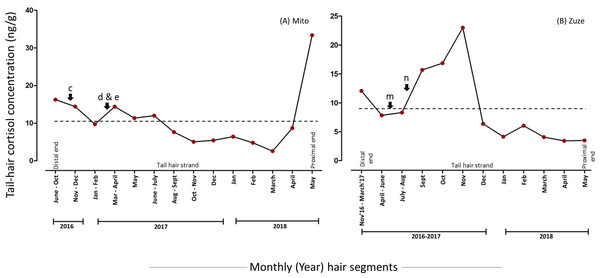
The overall mean hC level was 81.4% lower in the segments which were manually minced (10.88 (7.68) ng/g) than in powdered hair segments (58.55 (106.01) ng/g) of Mito (Fig. 1 & 2, Table 2, for detailed information refer to Supplementary Results). The hC levels in Mito ranged between 4.53 ng/g to 485.12 ng/g for the powdered samples and varied from 2.6 ng/g to 33.39 ng/g for the minced segments (Table 2). Even though the hC levels in Mito were lower in the minced samples, some of the patterns of hC during the biological events (events: c, d & e in Fig. 2 and Fig. 3; Table 2) were found to be similar in minced and powdered hair segments. When hC levels in powdered and minced samples (only for those months where hair segments were not combined) were compared, an average of 27.26% decline was observed (with decline ranging from −68.92% to −11.46%) in minced hC levels (Fig. S4). Wilcoxon signed rank exact test showed no significant differences between the two extraction methods for these months (V = 1, P = 0.063; n = 6 months; Fig. S4). For Zuze (KOZ), the hC was extracted only by mincing the hair segments and the overall mean hC level was 9.28 (6.31) ng/g varying from 3.43 ng/g to 23 ng/g (Fig. 3, Table 2; Supplementary Results).
Association between tail hC levels and biological events
The hC levels varied among and within individuals as well as over time (Table 2), with the mean hC levels ranging from 5.03 (7.58) ng/g in Bunnyun to 96.41 (43.22) ng/g in Kampart (Fig. 2). The highest hC level was observed in Mito (485.12 ng/g) and the lowest in Bunnyun (1.25 ng/g; Fig. 2, Table 2; Supplementary Results). Interestingly, when hC levels were compared with the elephant keepers’ daily records categorized as five potential stressors, some of the high and low values (based on the preceding month) were found to be associated with these stressors (Figs. 2 & 3, Table 2; Supplementary Results).
Levels of hC and psychosocial stressors
The hair segments corresponding to the psychosocial stressors (represented as a, b, m, and n in Figs. 2 & 3, Table 2) in the study animals had an average of 4.93-fold increase in the hC levels. The tail-hair segments corresponding to the months of July, August and September, 2016, showed a 14.26-fold increase in hC levels from 6.58 ng/g to 100.45 ng/g in Mito (Fig. 2, Table 2; Table S2; Supplementary Results). During these periods, the records indicated that Mito repeatedly showed reluctance to enter her enclosure and there were some pathological symptoms in her (Table 2). Similarly, Zuze’s minced hair samples showed hC levels of 7.85 ng/g (hair segments corresponding to April–June 2017) when her calf was brought back to KOZ and 88.42% increase to 15.70 ng/g when the calf was taken back to his previous zoo (hair segments corresponding to July–September 2017; Fig. 3, Table 2; Table S2; Supplementary Results). The hC level in Zuze’s tail-hair segments, after this event, continued to increase, for three months, until it reached the maximum value of 23.00 ng/g during November, 2017 and then dropped (Fig. 3; Table S2; Supplementary Results). Other than the records of her calf, there were no other unique biological events recorded for Zuze.
| S.No. | Study animal | hC levels (ng/gm) | hC levels corresponding to biological events | ||||
|---|---|---|---|---|---|---|---|
| Minimum | Mean (SD) | Maximum | Date (refer Figs. 1 & 2) | hC levels (ng/gm) | Stressors: Biologically ‘stressful’ events | ||
| I | Mito | 4.53 | 58.55 (106.01) | 485.12 | (a) July-2016 | 6.58 | Psychosocial: Reluctant behaviour towards entering her enclosure |
| (b) Aug to Sept-2016 | 5.92 to 100.45 | Psychosocial: Reluctant behaviour in entering the enclosure; Anaemic | |||||
| Pathological: Bleeding toe; antibiotic and trimming treatment | |||||||
| Social: Interaction with four elephants through fence; slight aggression | |||||||
| (c) Nov to Dec-2016 | 101.57 to 205.27 | Pathological: Symptoms of colic infection (along with anorexia) | |||||
| (d) Jan to March-2017 | 45.91 to 485.12 | Pathological: After effects of colic infection | |||||
| Social: Interaction with four elephants | |||||||
| (e) April-2017 | 60.09 | Practice: Semi-protected (early to mid of April) and protected contacts trial (Late April) with 4 elephants | |||||
| II | Tonkun | 10.06 | 53.62 (65.96) | 166.72 | (f) Nov-2017 | 166.72 | Reproductive: Observed signs of ‘estrus’ in early November |
| Pathological: Skin defect observed on the nostril septum | |||||||
| III | Bunnyun | 1.25 | 5.03 (7.58) | 20.45 | (g) Nov-2017 | 20.45 | Reproductive: ‘Followed’ by Tonkamu and attempts of mounting |
| IV | Kampart | 42.04 | 96.41 (43.22) | 151.81 | (h) Dec-2017 to Jan-2018 | 127.10 to 151.81 | Pathological: Oral sores |
| Peri-anal lesion (diameter = 0.5 cm) | |||||||
| (i) Jan to Feb-2018 | 151.81 to 81.30 | Practice: Training for 5 individuals to share the space | |||||
| (j) March-2018 | 79.82 | Pathological: Skin abrasion and slight swelling on left forelimb | |||||
| V | Tonkamu | 7.15 | 36.27 (39.47) | 131.21 | (k) Oct to Nov- 2017 | 131.21 to 25.57 | Reproductive/Social: ‘Following’ behaviour towards females |
| (l) Jan to Feb- 2018 | 13.90 to 23.96 | Pathological: Nostril infection | |||||
| VI | Mito (minced samples) | 2.6 | 10.88 (7.68) | 33.39 | (c) Nov to Dec-2016 | 14.41 | Pathological: Symptoms of colic infection (along with anorexia) |
| (d & e) March to April- 2017 | 9.74 to 14.40 | Pathological: After effects of colic infection | |||||
| Social: Interaction with four elephants | |||||||
| Practice: Semi-protected (early to mid of April) and protected contact trials (Late April) with 4 elephants | |||||||
| VII | Zuze (minced samples) | 3.43 | 9.28 (6.31) | 23.00 | (m) April to June-2017 | 7.85 | Psychosocial: Her calf was brought to Kobe Oji Zoo |
| (n) July to Sept-2017 | 8.33 to 15.70 | Psychosocial: Her calf was transferred back to the previous zoo. | |||||
Figure 2: Tail-hair cortisol concentrations (hC; ng/g of powdered hair samples) in five captive elephants of the Kyoto City Zoo.
Time series plots represent the month-wise (year) tail hC levels (on the y-axes) in (A) Mito (a 48-year old female), (B) Tonkun (a 10-year old female), (C) Bunnyun (a 8-year old female), (D) Kampart (a 8-year old female) and (E) Tonkamu (a 7-year old male) corresponding to the potential biological events, i.e., psychosocial (a, b), pathological (b, c, d, f, h, j, l), social (b, d, k), practice-related (e, i) and reproductive (f, g, k) stressors (refer to Table 1 for detailed descriptions). The scales of y-axes vary for each elephant. Arrowhead and a line represent the direction of proximal to distal ends of a tail-hair strand. A dashed line represents the overall mean value of hC levels for an individual.Levels of hC and pathological stressors
The months corresponding to psychosocial stressors (represented as b, c, d, f, h, j and l in Figs. 2 & 3, Table 2) in the study animals showed an average of 3.59-fold increase in the hC levels. Pathological stressors, such as bleeding toe, anaemia, colic infection and anorexia as reported by the keepers, elevated the hC levels in corresponding tail-hair segments of Mito (in the powdered samples, b: 15.97-fold increase from 5.92 ng/g to 100.45 ng/g; c: 1.02-fold increase from 101.57 ng/g to 205.27 ng/g and d: 9.57-fold increase from 45.91 to 485.12 ng/g and in the minced samples, c: 14.40 ng/g and 47. 8% increase from 9.74 ng/g to d & e: 14.41 ng/g; Figs. 2 & 3, Table 2; Table S2; Supplementary Results). Similarly in Tonkun, the level of hC was found to be 166.72 ng/g (a highest value recorded for Tonkun; 1.91-fold higher than the hC level in the following month: 57.26 ng/g) when she had skin defects on the nostril (along with this, she showed signs of estrus; Fig. 2 (event: f), Table 2; Supplementary Results). Kampart’s tail-hair segments showed 19.4% increase in hC levels from 127.10 ng/g to 151.81 ng/g when she suffered from oral sores and peri-anal lesion, and 79.82 ng/g when she had skin abrasions and limb swelling (47.3% higher than the hC level (42.04 ng/g) in the following month; Fig. 2 (events: h and j), Table 2, Table S2; Supplementary Results). In Tonkamu, the hC levels in the corresponding hair-segments were found to increase by 72.4% from 13.90 ng/g to 23.96 ng/g when he suffered from nostril infection (event: l; Fig. 2, Table 2; Table S2; Supplementary Results).
Figure 3: Tail-hair cortisol concentrations (hC; ng/g of manually minced hair samples) in two captive elephants of the Kyoto City Zoo and Kobe Oji Zoo.
Time series plots represent the month-wise (year) tail hC levels in (A) Mito and (B) Zuze corresponding to the important biological events viz. pathological (c, d), practice-related (e) and psychosocial (m, n) stressors (refer to Table 1 for detailed descriptions). The scales of y-axes vary for each elephant. A dashed line represents the overall mean value of hC levels for an individual.Levels of hC and social stressors
An average of 11.9-fold increase was observed in hC levels of Mito’s hair segments corresponding to social stressors (represented as b and d in Figs. 2 & 3, Table 2). However, events related to social stressors (such as interaction with four new elephants) were only reported in Mito and these stressors were found to be recorded along with other psychosocial and pathological events. Thus, the levels of hC were always found to be higher in Mito’s hair segments during these events (refer to Figs. 2 & 3 (events: b & d), Table 2; Table S2; Supplementary Results). Similarly, there was one event (Table 2, event: k) where 80.5% decline in hC levels was observed for Tonkamu’s ‘following’ behaviour towards two females; however, we marked it as a reproductive stressor (as mounting attempts were recorded).
Levels of hC and reproductive stressors
The hC levels related to reproductive stressors were found to be higher as 75.5% decline was observed on average in the hC levels in the hair segments in the months following reproductive events (represented by: f, g, and k in Fig. 2, Table 2). Hair segments corresponding to the month of November-2017, the hC level in Tonkun was found to be at the highest, i.e., 166.72 ng/g and, during this period, she showed signs of estrus (however, there were also pathological symptoms of infection in the skin of her nostril during the same period; Fig. 2, Table 2; Table S2; Supplementary Results). A decline of 65.7% was observed in hC levels in the following month (166.72 ng/g to 57.26 ng/g) in Tonkun. Similarly, Bunnyun had a 20.45 ng/g hC level (which declined by 85.3% to 3.01 ng/g in the following month) when Tonkamu ‘followed’ and attempted to mount her and Kampart (Table 2). In the case of Tonkamu, who followed two females and attempted to mount them, the hC levels declined by 80.5% from 131.21 ng/g to 25.57 ng/g (Fig. 2, Table 2; Table S2; Supplementary Results).
Levels of hC and husbandry-practice related stressors
An average of 67.0% decline was observed in hC levels corresponding to husbandry practice-related stressors (represented as e and i in Figs. 2 & 3, Table 2). Semi-protected, protected contact trials and sharing of space between Mito and other four new elephants at the KCZ lowered down the hC levels in Mito by 87.6% (from 485.12 ng/g to 60.10 ng/g) and in Kampart by 46.5% (151.81 ng/g to 81.30 ng/g; Fig. 2, Table 2; Table S2; Supplementary Results).
Besides these, there were other higher and lower peaks in hC levels observed in some of the individuals; however, there were no biological records reported during these periods (Figs. 2 & 3). Similarly, there were very few biological records (mainly pathological), where peaks in hC levels were not observed. For the detailed results for each individual refer to Supplementary Results.
Discussion
This study, for the first time, shows that tail-hair in elephants has the potential to be an alternative index to measure cortisol and decipher an individual’s physiological history. In addition, some of the observed associations between the hC levels and biological events, such as higher hC levels during pathological and psychosocial stress and lower hC levels during husbandry-practices, suggest the possibility of elephants’ tail-hair being a ‘retrospective’ calendar of their well-being.
There are numerous studies on elephant hair addressing varied topics such as hair and protein structures in mammoth (Gillespie, 1970; Valente, 1983; Hillary & Buys, 1984), reliability of hair-follicle mitochondrial DNA (Greenwood & Pääbo, 1999), genetic variation in hair length candidate genes (Roca et al., 2009), the migration patterns, chronologies, dietary history and seasonal dietary shift through stable isotopes from tail-hair (Cerling et al., 2004; Cerling et al., 2006; Cerling et al., 2009; Wittemyer, Cerling & Douglas-Hamilton, 2009; Uno et al., 2020), forensic species identification (Yates, Espinoza & Baker, 2010), thermo-regulatory properties of body hair (Myhrvold, Stone & Bou-Zeid, 2012), trace element concentrations (Hu, Fernandez & Cerling, 2018), tensile strength of hair (Yang et al., 2019) and scent-flagging behaviour (Raha et al., 2013). From existing evidence, there is no study that assessed the physiological markers in the hair of elephants. This study, thus, attempted to quantify the cortisol marker in the tail-hair segments.
In this study, the growth rates of the tail hairs in captive Asian elephants were between 0.40 mm/day and 0.67 mm/day as compared to between 0.73 mm/day and 1.04 mm/day in free-ranging African elephants (Cerling et al., 2009). Inter-individual variation was observed in the hair growth rate, but differences in growth rate between hairs within the same individual were minimal (except for Mito). Hair growth in humans and other animals has been shown to be greatly influenced by a variety of exogenous and endogenous factors including quality of diet (nutritional contents such as zinc and iron status - Rushton, 2002), seasonality (Randall & Ebling, 1991), environmental factors (temperature, pollution - Johnson, 1981; Horev, 2007), age and sex of an individual (Bertoli et al., 2020) and their physiological and reproductive states (Randall, 2008; Grymowicz et al., 2020). There is a paucity of scientific information on how these factors influence the hair growth rates in elephants. A longitudinal study including the variations in hair growth within/between individuals, between months, over the seasons, reproductive states, environmental and dietary influences is highly recommended.
The overall mean and standard deviations of hC levels in Mito were higher for the powdered samples than that of minced samples. This could be due to some of the peak values being two to 15-fold higher than the overall mean in powdered samples and due to poor extraction and low detectability in minced samples. Although the powdered samples showed higher values than that of minced ones suggesting that grinding may facilitate better extraction and analyses of hC (Yamanashi, 2018), differences observed were not statistically significant. As the comparisons were done only for Mito’s tail-hair segments, increasing hair samples from more individuals may aid in further validating the extraction technique. Most of the thick tail-hair samples were hard to pulverize and upon pulverization, they appeared ‘greasy’ with peculiar odor. This could be due to the presence of fatty acids and malodorous compounds in the tail-hair of elephants as suggested by Raha et al. (2013).
The measured hC levels showed clear associations with distinct biological events recorded by the keepers when compared a posteriori, indicating that the tail-hair conceivably can be used to assess the history of ‘stress’ in elephants. For instance, in the case of Mito, we observed the increased percentage change of hC levels around the segments relating to psychosocial and pathological stressors and decline around the segments with practice-related and interaction events. In the case of Tonkun, Kampart and Tonkamu, the increased percentage of hC levels corresponded with pathological conditions such as lesions, infections and oral sores. Bunnyun, Kampart and Tonkamu showed the behaviours which could be linked to psychosocial or reproductive stressors, where the peak hC level was observed in Bunnyun and lower in Tonkamu who was following these two females. The ‘following’ behaviour, as recorded in the keepers’ diary, of Tonkamu (male) could be for social interactions with these females or could be triggered by the onset of reproductive maturity because he persistently followed Bunnyun and showed mounting behaviour which is considered as a sign of social facilitation, social interaction or sexual activity (Rees, 2004). The lowered hC levels, thus, could be the resultant of such social interactions. The patterns in decline and a sharp increase in hC levels in Zuze’s tail-hair could be linked with psychosocial stress due to the arrival of her calf and his transfer to the previous zoo, respectively. The husbandry-practice related events, such as contact trials and trainings to share the space, showed lower hC levels in the study animals. Similar associations between these stressors (such as infections, diet, social bonding, body condition) and faecal glucocorticoid metabolites have been well-established for wild Asian and African elephants (Ganswindt et al., 2010; Pokharel, Seshagiri & Sukumar, 2017; Pokharel, Seshagiri & Sukumar, 2020; Pokharel et al., 2019). These qualitative comparisons indicate the prospective utility of a tail-hair of an elephant as a retrospective calendar of its physiological past.
Several potentially confounding factors need to be tested before considering the tail-hair as a retrospective scale of physiological details. Some of them include the segmental analysis of cortisol, incorporation pathway of cortisol in hair, biases due to the contamination and local production of cortisol by sweat and sebaceous glands, and the leaching effect or washout effect of cortisol from proximal to distal ends (Kirschbaum et al., 2009; Russell et al., 2012; Keckeis et al., 2012; Yamanashi, 2018; Heimbürge, Kanitz & Otten, 2019; Kalliokoski, Jellestad & Murison, 2019). The findings from this study, highlighting the associations between hC levels and biological events, suggest that cortisol may be segmentally deposited in hair. As the tail-hair of elephants have already been used to assess the dietary history, migration patterns and chronology through segmental analyses of tail-hair based on its growth rate (Cerling et al., 2004; Cerling et al., 2006; Cerling et al., 2009; Wittemyer, Cerling & Douglas-Hamilton, 2009), findings from this study further supports the potentiality of studying the physiological histories through hair segments.
Unlike earlier suggestions that cortisol in hair may leach out from the proximal to the distal ends (Russell et al., 2012; Yamanashi, 2018; Heimbürge, Kanitz & Otten, 2019; Kalliokoski, Jellestad & Murison, 2019), in this study the patterns of hC levels varied across segments of a hair strand, without any gradual decline in hC levels from the proximal segments to the tip of the hair strands. This evidence may rule out the existence of the “washout” effect in elephant tail-hair. While it is debated that the local production of hC levels by sudoriferous (sweat) and sebaceous (sebum-secreting) glands in other animal species may affect the overall hC levels (Pragst & Balikova, 2006; Russell et al., 2012; Salaberger et al., 2016; Yamanashi, 2018; Heimbürge, Kanitz & Otten, 2019), extant elephants lack sebum and sweat glands (Wright & Luck, 1984; Lillywhite & Stein, 1987). Although the presence of sebaceous glands were reported in the extinct woolly mammoth (Repin et al., 2004), and temporal and interdigital glands in extant elephants are considered as the modified apocrine and eccrine sweat glands, respectively (Fowler & Mikota, 2006), the existence of true sweat and sebaceous glands in elephants has not yet been determined. Thus, the contribution of the hC produced locally through these glands may be negligible in the case of elephants.
The study measured the hC levels in the tail-hair shafts. However, how the follicular HPA axis would influence the hC levels (either in the hair shafts or the whole hair) in elephants should be examined (Ito et al., 2005; Russell et al., 2012; Heimbürge, Kanitz & Otten, 2019; Sergiel et al., 2020). The findings of the current study clearly suggest that the tail-hair of elephants can be one of the important biological matrices to reconstruct the physiological history of both wild and captive elephants.
Conclusions
This study opens an avenue to further explore the use of tail-hair to assess stress at the retrospective scale and to address broader ecological questions in the life of an individual animal. Though in an exploratory state, our findings indicate the possibility of assessing cortisol in hair to understand the physiological history of elephants. These findings and interpretations can be further improved by enhancing the sampling effort (adding more samples and controls), adapting different extraction techniques, analytically (recovery and extraction efficiency analyses), physiologically and biologically validating the incorporation of cortisol into the hair shaft (Kapoor, Schultz-Darken & Ziegler, 2018), integrating additional predictor variables (variations due to age, sex, season, diet, reproductive phases), and assessing the influence of within-individual variation (variations due to hair type: body hair or tail-hair, hair-textures). Further comparisons of cortisol levels and establishment of correlations in different matrices (faecal, urine, blood and hair samples) in elephants are highly recommended. The utility of hC and other biomarkers in hair can help to assess the influence of various captive conditions (such as housing condition, social grouping, diet, disease or pathological states) and, in the case of wild elephants, in deciphering the ecological history of a deceased individual (or an animal captured for research or other purposes) over the last few years. Further, analyzing hC levels along with other biomarkers (such as reproductive hormones) can facilitate more meaningful biological interpretations of an animal’s life.
Supplemental Information
Study animals from Kyoto City and Kobe Oji Zoos
Photographs of the elephants from whom the tail-hair samples were collected. Photo credits: Kyoto City Zoo and Kobe Oji Zoo.
Mincing technique used for cortisol extraction
An illustration of the mincing technique adopted for extraction of tail-hair cortisol. Monthly hair segments were manually and uniformly minced as close as the individual daily tail-hair growth rate (Illustrated by Sanjeeta Sharma Pokharel).
Parallelism between serially diluted tail hair cortisol and standards
Graph showing parallelism between serial dilutions (ratios starting from 1:2 to 1:16) of a tail hair extract (marked as ‘Hair samples’) from one of study animals and standards of the cortisol enzyme-linked immunosorbent assay (EIA) with FKA404E antibody and FKA403 antigen (Cosmo Bio Co., Ltd.) fitted with 4-parameters fit (r2 = 0.997) using the MPM 6 software.
Tail-hair cortisol levels in powdered vs. minced hair segments in Mito
The lines represent the hC levels in Mito’s tail-hair segments extracted using powdered (red circle) and minced (black square) samples corresponding to a few selected months (months in which hair segments were not pooled). The negative percentage represents the percentage decline in hC levels in the minced hair segments. The boxplot in the inset (i) shows no significant difference (Wilcoxon V = 1, P = 0.063) in median hC levels extracted using minced and powdered hair segments.
Raw data showing the tail hair growth rate in all six captive elephants
Initial readings of hair length after cut, final readings after 18 days (*except for Zuze: hair length was measured in 14 days), total growth rate (TGR) per 18 days (*14 days for Zuze), TGR per day and per month along with standard deviations (SD).
Raw data showing the tail-hair cortisol levels in all six captive elephants (when powdered and minced extracts were used)
The month-wise hC levels for each individual (for both powdered and minced samples) and values marked in bold are minimum and maximum levels of hC in each individual.For the minced samples, if the combined months exceeded 3 months*, such values were not used for a-posteriori comparisons.