Abstract
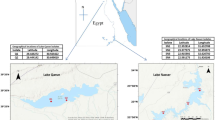
One strain of unicellular greenish algae embedded by mucilage was successfully isolated from equatorial area in the Indian Ocean. Microscopic observation, ultrastructure features and genetic identification confirmed that the strain was closely related to Cyanothece sp., which was a cyanobacteria species with great ecological significance. Cells were solitary with oval or bacilliform shapes. Diameters of this strain were relatively small, ranging from 2.5 to 6.5 μm on average. Ultrastructure of cells was simple. Thylakoids were arranged parietal and keritomized content were observed in the thylakoid region. Various electron-transparent granules with low electron-dense region as well as cyanophycin or glycogen granules-like organelle and carbonxysomes were also observed. For pigment composition, the dominant pigments were chlorophyll a, β-Carotene, Zeaxanthin and an unknown pigment, contributing 23.8%, 26.1%, 14.7% and 15.7% to total pigments respectively. The phylogenetic analysis of 16S rRNA gene and nifH gene confirmed that Strain EIO409 was closely related with Cyanothece sp..
Similar content being viewed by others
References
Bandyopadhyay A, Elvitigala T, Welsh E, et al. 2011. Novel metabolic attributes of the genus Cyanothece, comprising a group of unicellular nitrogen-fixing cyanobacteria. mBio, 2(5): e00214–11
Castenholz R W, Wilmotte A, Herdman M, et al. 2001. Phylum BX. Cyanobacteria. In: Boone D R, Castenholz R W, Garrity G M, eds. Bergey’s Manual® of Systematic Bacteriology. New York: Springer, 473–599
Cepák V. 1993. Morphology of DNA containing structures (nucleoids) as a prospective character in cyanophyte taxonomy. Journal of Phycology, 29(6): 844–852, doi: 10.1111/j.0022-3646.1993.00844.x
Cohen Y, Padan E, Shilo M. 1975. Facultative anoxygenic photosynthesis in the cyanobacterium Oscillatoria limnetica. Journal of Bacteriology, 123(3): 855–861
Dor I. 1998. A checklist of cyanophyta (cyanobacteria) of Israel and adjacent regions. Israel Journal of Plant Sciences, 46(3): 239–254, doi: 10.1080/07929978.1998.10676733
Fine R A, Smethie W M Jr, Bullister J H, et al. 2008. Decadal ventilation and mixing of Indian Ocean waters. Deep Sea Research Part I: Oceanographic Research Papers, 55(1): 20–37, doi: 10.1016/j.dsr.2007.10.002
Galloway J N, Dentener F J, Capone D G, et al. 2004. Nitrogen cycles: past, present, and future. Biogeochemistry, 70(2): 153–226, doi: 10.1007/s10533-004-0370-0
Garcia-Pichel F, Nübel U, Muyzer G. 1998. The phylogeny of unicellular, extremely halotolerant cyanobacteria. Archives of Microbiology, 169(6): 469–482, doi: 10.1007/s002030050599
Garrity G, Boon D R, Castenholz R W. 2001. Bergey’s Manual of Systematic Bacteriology: Volume 1. the Archaea and the Deeply Branching and Phototrophic Bacteria. 2nd ed. New York: Springer
Guillard R R L, Ryther J H. 1962. Studies of marine planktonic diatoms: I. Cyclotella nana Hustedt, and Detonula conferraceae (Cleve) gran. Canadian Journal of Microbiology, 8(2): 229–239, doi: 10.1139/m62-029
Hayes P K, El Semary N A, Sánchez-Baracaldo P. 2007. The taxonomy of cyanobacteria: molecular insights into a difficult problem. In: Brodie J, Lewis J, eds. Unravelling the Algae: The Past, Present, and Future of Algal Systematics. Boca Raton, FL: CRC Press
Komárek J. 1976. Taxonomic review of the genera Synechocystis Sauv. 1892, Synechococcus Nag. 1849, and Cyanothece gen. nov. (Cyanophyceae). Archiv für Protistenkunde, 118: 119–179
Komárek J, Cepák V. 1998. Cytomorphological characters supporting the taxonomic validity of Cyanothece (Cyanoprokaryota). Plant Systematics and Evolution, 210(1–2): 25–39, doi: 10.1007/BF00984725
Komárek J, Cepák V, Kaštovský J, et al. 2004. What are the cyanobacterial genera Cyanothece and Cyanobacterium? Contribution to the combined molecular and phenotype taxonomic evaluation of cyanobacterial diversity. Algological Studies, 113(1): 1–36, doi: 10.1127/1864-1318/2004/0113-0001
Komárek J, Kopecký J, Cepák V. 1999. Generic characters of the simplest cyanoprokaryotes Cyanobium, Cyanobacterium and Synechococcus. Cryptogamie Algologie, 20(3): 209–222, doi: 10.1016/S0181-1568(99)80015-4
Kumar S P, Narvekar J, Nuncio M, et al. 2009. What drives the biological productivity of the Northern Indian Ocean?. In: Wiggert J D, Hood R R, Naqvi S W A, et al., eds. Indian Ocean Biogeochemical Processes and Ecological Variability. Washington, DC: American Geophysical Union, 33–56
Lee R E. 2008. Phycology. 4th ed. Cambridge: Cambridge University Press, 547
Margheri M C, Ventura S, Kaštovský J, et al. 2008. The taxonomic validation of the cyanobacterial genus Halothece. Phycologia, 47(5): 477–486, doi: 10.2216/07-87.1
Mikhodyuk O S, Gerasimenko L M, Akimov V N, et al. 2008. Ecophysiology and polymorphism of the unicellular extremely natronophilic cyanobacterium Euhalothece sp. Z-M001 from Lake Magadi. Microbiology, 77(6): 717–725, doi: 10.1134/S0026261708060106
Mogany T, Swalaha F M, Allam M, et al. 2018. Phenotypic and genotypic characterisation of an unique indigenous hypersaline unicellular cyanobacterium, Euhalothece sp. nov.. Microbiological Research, 211: 47–56, doi: 10.1016/j.micres.2018.04.001
Nübel U, Garcia-Pichel F, Muyzer G. 1997. PCR primers to amplify 16S rRNA genes from cyanobacteria. Applied and Environmental Microbiology, 63(8): 3327–3332
Oren A. 2009. Problems associated with the taxonomic validation of the cyanobacterial genus Halothece by Margheri et al. 2008, Phycologia 47: 477–486. Phycologia, 48(4): 313–314, doi: 10.2216/09-33.1
Park J W, Nam S W, Kim H S, et al. 2014. Enhanced photobiological H2 production by the addition of carbon monoxide and hydrogen cyanide in two unicellular N2-fixing cyanobacterial strains isolated from Korean coasts. Ocean Science Journal, 49(1): 11–18, doi: 10.1007/s12601-014-0002-0
Reddy K J, Haskell J B, Sherman D M, et al. 1993. Unicellular, aerobic nitrogen-fixing cyanobacteria of the genus Cyanothece. Journal of Bacteriology, 175(5): 1284–1292, doi: 10.1128/jb.175.5.1284-1292.1993
Reddy K J, Soper B W, Tang J, et al. 1996. Phenotypic variation in exopolysaccharide production in the marine, aerobic nitrogen-fixing unicellular cyanobacterium Cyanothece sp.. World Journal of Microbiology and Biotechnology, 12(4): 311–318, doi: 10.1007/BF00340206
Rippka R, Cohen-Bazire G. 1983. The cyanobacteriales: a legitimate order based on the type strain Cyanobacterium stanieri?. Annales de l’Institut Pasteur/Microbiologie, 134(1): 21–36
Rixen T, Ramaswamy V, Gaye B, et al. 2009. Monsoonal and ENSO impacts on particle fluxes and the biological pump in the Indian ocean. In: Wiggert J D, Hood R R, Naqvi S W A, et al., eds. Indian Ocean Biogeochemical Processes and Ecological Variability. Washington, DC: American Geophysical Union, 365–383
Roussomoustakaki M, Anagnostidis K. 1991. Cyanothece halobia, a new planktic chroococcalean cyanophyte from Hellenic heliothermal saltworks. Algological Studies/Archiv für Hydrobiologie, (64): 71–95
Rudi K, Skulberg O M, Larsen F, et al. 1997. Strain characterization and classification of oxyphotobacteria in clone cultures on the basis of 16S rRNA sequences from the variable regions V6, V7, and V8. Applied and Environmental Microbiology, 63(7): 2593–2599
Spurr A R. 1969. A low-viscosity epoxy resin embedding medium for electron microscopy. Journal of Ultrastructure Research, 26(1–2): 31–43, doi: 10.1016/S0022-5320(69)90033-1
Tamura K, Peterson D, Peterson N, et al. 2011. MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Molecular Biology and Evolution, 28(10): 2731–2739, doi: 10.1093/molbev/msr121
Wang Jing, Kan Junjun, Zhang Xiaodong, et al. 2017. Archaea dominate the ammonia-oxidizing community in deep-sea sediments of the eastern Indian ocean—from the equator to the bay of Bengal. Frontiers in Microbiology, 8: 415
Waterbury J B, Watson S W, Valois F W. 1986. Biological and ecological characterization of the marine unicellular cyanobacterium Synechococcus. Canadian Bulletin of Fisheries and Aquatic Sciences, 214: 71–120
Welsh E A, Liberton M, Stöckel J, et al. 2008. The genome of Cyanothece 51142, a unicellular diazotrophic cyanobacterium important in the marine nitrogen cycle. Proceedings of the National Academy of Sciences of the United States of America, 105(39): 15094–15099, doi: 10.1073/pnas.0805418105
Zapata M, Rodríguez F, Garrido J L. 2000. Separation of chlorophylls and carotenoids from marine phytoplankton: a new HPLC method using a reversed phase C8 column and pyridine-containing mobile phases. Marine Ecology Progress Series, 195: 29–45, doi: 10.3354/meps195029
Zehr J P, Mellon M T, Zani S. 1998. New nitrogen-fixing microorganisms detected in oligotrophic oceans by amplification of nitrogenase (nifH) genes. Applied and Environmental Microbiology, 64(9): 3444–3450
Zhang Yunyi, Chi Zhenming, Lu Weidong. 2007. Exopolysaccharide production by four cyanobacterial isolates and preliminary identification of these isolates. Journal of Ocean University of China, 6(2): 147–152, doi: 10.1007/s11802-007-0147-x
Author information
Authors and Affiliations
Corresponding author
Additional information
Foundation item: The National Natural Science Foundation of China under contract Nos 41876134, 41676112, 41276124, 41406155 and 41506182; the Natural Science Foundation of Tianjin under contract No. 17JCZDJC40000; the Exploration Program of Ocean with Science and Technology of Tianjin under contract No. KJXH2013-22; the Changjiang Scholar Program of Chinese Ministry of Education of China to Jun Sun.
Rights and permissions
About this article
Cite this article
Zhang, X., Yang, S., Sun, J. et al. Morphology, ultrastructure and phylogeny of Cyanothece sp. (Cyanobacteriaceae: Cyanophyceae) isolated from the eastern Indian Ocean. Acta Oceanol. Sin. 37, 4–10 (2018). https://doi.org/10.1007/s13131-018-1297-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s13131-018-1297-y