Abstract
Shallow waters, little-studied in Continental Antarctica, among other micrometazoans host bdelloid rotifers, which diversity, ecology, and distributional patterns in turn are poorly known. To address these issues, we analysed plankton samples collected during the 2018/2019 season in the Thala Hills oasis (East Antarctica), in shallow freshwater lakes and temporary ponds that formed during intense snow melting in December–January. Bdelloids were present in more than 90% of the sites with nine species revealed. The most frequent were Antarctic endemics [Philodina gregaria (P. gregaria), Adineta grandis (A. grandis), and Adineta coatsi (A. coatsi)], while some non-abundant bdelloids either provide characteristics of widely distributed taxa or require further taxonomy studies as they can be species new for the science. The abundance of bdelloids varied greatly across studied sites and localities, with a maximum of more than 700,000 ind m−3 and an increasing tendency to be more numerous in rock-basin temporary ponds, compared to larger lakes, with variability for different taxa. The environmental parameters strongly explain the bdelloid distribution (78.4% of the variation), with the most important factors being the type of bottom (9.9%), altitude (8.0%), TDS (6.6%), and salinity (6.5%). The cyanobacterial mats from the bottom didn’t contribute much to bdelloid distributional patterns, despite being known to be a preferred habitat for micrometazoans including rotifers. These results shape a perspective to study the processes of the formation of Antarctic seasonal aquatic habitats settled by organisms, which demonstrate an ecomorphological range from planktonic organisms to crawling ‘scrapers’.
Similar content being viewed by others
Introduction
Low biological diversity and truncated food chains with a lack of high trophic levels are important characteristics of the freshwater ecosystems in the Continental Antarctic (Laybourn-Parry et al. 1997; Camacho 2006; Gibson et al. 2006; Laybourn-Parry and Pearce 2007). Consisting of communities of organisms that are adapted for existence in harsh conditions (Vincent 1988, 2000; Convey and Stevens 2007; Rogers et al. 2012; Laybourn-Parry and Wadham 2014), they can be considered models for evolutionary and ecology studies (Simmons et al. 1993; Laybourn-Parry and Pearce 2007; Camacho et al. 2012), which are becoming more important as the Antarctic environment is facing global changes (Convey 2010; Chown et al. 2012; Convey and Peck 2019).
To date, most studies of freshwater ecosystems in the Continental Antarctic have been carried out at large stratified lakes and have focused mostly on auto- and heterotrophic microbial communities (e.g., Ellis-Evans 1996; Laybourn-Parry et al. 1996, 1997; Vyverman et al. 2010; Rochera and Camacho 2019; Izaguirre et al. 2021). Shallow waters, neglected globally from scientific point of view (Bégin and Vincent 2017), have remained largely unexplored in the Antarctic as well, leaving organisms, populations and communities little-known.
All metazoans in water bodies in Continental Antarctica are presented by organisms less than a few millimetres and even of microscopic sizes: rotifers, tardigrades, nematodes, crustaceans, gastrotrichs, and flatworms (Dartnall 2017; Díaz et al. 2019). The first ones are one of the most essential parts of both metazooplankton and benthic communities and can be the top consumers in the absence of crustaceans (Camacho 2006). Despite the accumulation of significant knowledge on rotifer diversity and distribution in the Antarctic (generally referenced in Sudzuki 1964; Fontaneto et al. 2015; Dartnall 2017), obligatory parthenogenetic bdelloids (subclass Bdelloidea), which cannot be identified in chemically preserved samples, have barely been studied. A limited number of works have indicated Bdelloidea species in freshwater ecosystems of Continental Antarctica (e.g., Kutikova 1991; Dartnall 1995, 2000; Hansson et al. 2012), whilst up-to-date taxonomical studies of bdelloids in terrestrial and limno-terrestrial systems have opened up prospects for research on this group of organisms in the Antarctic (Iakovenko et al. 2015).
Long-standing monitoring during the 2009–2016 seasons, conducted at shallow water bodies in the eastern part of the Thala Hills oasis (Enderby Land, Eastern Antarctica), has shown preliminary indications that bdelloid rotifers constitute a major part of the zooplankton (Lukashanets et al. 2019, 2021). Despite their high occurrence frequency and abundance, they have mostly remained unidentified as only regular hydrobiological procedures were used. In the present study, a detailed description was made of bdelloid assemblages in shallow waters at the Thala Hills, with the following aims in mind: (i) increasing knowledge about the taxonomic diversity of Antarctic rotifers, with data obtained at the Thala Hills, which is the hitherto poorly studied and remote area; (ii) elucidating the main biases of bdelloids in relation to water (pH, dissolved oxygen and conductivity, etc.) and other environmental parameters (altitude, bottom type); and (iii) clarification of the role of cyanobacterial bottom mats (hereinafter referred to as CBM), which have been identified as a precondition for bdelloids emerging. These mats were hypothesized to be an attractive habitat, from which organisms can emerge and enter the water column. Samples collected between 2009 and 2016 have frequently indicated presence of many tardigrades (which are completely non-plankton metazoans), presumably associated with bottom particles, suspended or stirred in the water (e.g., as a result of sampling manipulations). Bdelloid rotifers tend to be ‘crawlers’ rather than ‘plankters’, and, in the Antarctic, are also known to be associated with benthic mats (Gibson et al. 2006; Wada et al. 2022). Therefore, it was discussed whether bdelloids emerging in the water were caused by bottom mats and stirring processes or did not depend on these.
Material and methods
Study area
All fieldwork was conducted in the eastern part of the Thala Hills oasis (Enderby Land, East Antarctica), near the Belarusian Antarctic Station ‘Vechernyaya Mount’ (hereinafter referred to as BAS). The study area is relatively low-lying and ice-free with granitoid rock surface mottled with depressions and riegels, which is a typical East Antarctic coastal oasis.
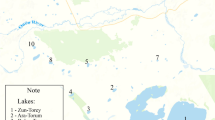
A total of 28 sites (water bodies) were sampled and divided into eight groups corresponding to localities separated spatially, by distance or relief: BAS area, Dostupny Cape, Northern Ridge, Southern Mount (216 m), Vechernyaya Mount (270 m), Unnamed Cape, Rubin Hill and Gnezdovoy Cape (Table 1, Fig. 1).
Five of the sampling sites were shallow but large and permanent water bodies and hence were considered as ‘lakes’. The others were temporary water ponds (pools), emerging at numerous terrain depressions, ground- and rock-basins during the intensive melting in December–January and are expected to be solid frozen during the winter (Fig. 2). Lake Nizhnee (BAS7) is the largest lake more than 100 m in length and 50 m in width. The extension of the other 27 water bodies ranges from 2 to 20 m. All sampling sites were snow-fed but varied greatly in bottom characteristics: ground, rock, silt-like soft sediments, or ‘ground/rock’ and ‘ground/soft’ combinations. Water bodies varied by the extensivity of the CBM, which sometimes totally cover the bottom (Fig. 3). We determined CBM as non-extensive if they occupy less than 30% of the bottom square, and as extensive—in case of 30–100% coverage.
Data collection
The plankton samples were collected from the water surface layer. The sampling at each site included two types of samples. The first ones, quantitative, were collected by means of standard hydrobiological procedures (Andrews 1972; Schwoerbel 1972) using plankton net (45-µm mesh diameter) filtering up to 200 L water. Samples were fixed using a 4% formalin solution. Qualitative samples were collected in the same places by plankton net with 20-µm mesh diameter, filtering 50–100 L water and were subsequently frozen at – 20 °C without chemical fixation. At six sites, we managed to obtain quantitative samples only.
In parallel with the sampling, we assessed the main physical and chemical parameters using Horiba U-50 multi-parameter water quality probe.
Sample processing
Rotifer individuals from the quantitative samples or, in case of huge densities, sample aliquots were counted at Petri dishes or counting chambers under dissection microscopy and expressed as a number of individuals per m3 (hereinafter referred to as ind∙m−3). While being in a contracted shape (‘tun’) after contacting with the formalin, they remained unidentified. Live rotifers from defrosted qualitative samples were identified under light microscopy. Appropriate chlorine-containing reagents were used to dissolve soft parts of rotifer individuals to make details of sclerotized jaws visible. To obtain body dimensions and proportions we performed the morphometric analyses as described by Iakovenko et al. (2013). Micrographs of bdelloids were obtained using NIKON E200 camera and NIS Elements BR analysis 5.10.00 software. As the present study focuses on the ecology and distributional patterns rather than taxonomy we consider ‘species’ as morphologically distinct, recognized entities. Expected cryptic diversity wasn’t the study subject.
Data analysis
The processing of quantitative samples provided values of abundances for Bdelloidea sp. (without dividing on smaller taxa). The abundance of tardigrades and nematodes, which were captured by plankton net, also was estimated.
The taxonomic diversity (species incidence data) was revealed from the qualitative samples. Alpha (α) diversity was calculated as the number of species per sample. Beta (β) diversity (the differences in species composition between sites) was estimated as Sørensen distance with building the pair-wise matrix. The pair-wise matrix of geographic distances between sites was also computed. The correlation of the geographical distances between sites and the species distances between these sites was computed by comparing two matrices with the Mantel test. The analysis of similarities ANOSIM was used to elucidate whether differences in species diversity between sites within one locality are lower than differences in species diversity between localities. Gamma (γ) diversity was calculated as the sum of the species overall at the study area. Apart from the observed number of species, the number of species that potentially can be found within the area was estimated using several methods: Chao algorithm, incidence-based coverage estimator (ICE), and Jackknife estimator. The following formulas from Gotelli and Colwell (2011) were used:
where SChao—number of species obtained by means of Chao algorithm; Sobs—observed number of species; m – number of samples; q1—number of species found in one sample; q2—number of species found in two samples;
where SICE—number of species obtained by means of ICE; Sfreq—number of frequent species (each found in more than 10 samples); Sinfreq—number of infrequent species (each found in 10 or fewer samples); CICE—sample coverage estimate (proportion of all incidences of infrequent species that are not unique); ninfreq—total number of incidences in the infrequent species; γ2 is the coefficient of variation;
where SJackknife—number of species obtained by means of Jackknife estimator.
When revealing the ecology and distributional patterns we operated with the appropriate abundance values of bdelloid species or some higher taxa, not Bdelloidea sp. The abundance values for species were computed as the product of (a) the proportion of the species (from 0 to 1) from qualitative samples and (b) total bdelloid abundance from quantitative samples. The Adineta genus was of significant importance as it can be considered as a group that quite likely could emerge in plankton samples from stirring up CBM, if we are basing on the morphology of genus members (corona is absent, large rake teeth for scraping). In that way, Adineta spp. were opposed to corona-bearing Philodinida spp., which can be both associated with CBM and be the component of the plankton in its traditional sense.
To analyze the differences in rotifer abundances across various groupings (e.g., bottom type, localities, CBM extensivity) we used the non-parametrical methods of comparing two (Mann–Whitney U-test) or several datasets (Kruskal–Wallis test). To show how bdelloid species disperse along the variation of the environmental parameters (water chemistry, altitude, type of the bottom, CBM), constrained ordination by means of redundant analysis, or RDA was used (Zuur et al. 2007; Paliy and Shankar 2016).
R Statistical Software (v4.2.1; R Core Team 2021) was used for graph plotting, performing all statistical tests (packages ‚geosphere’—Hijmans 2021, and ‘vegan’—Oksanen et al. 2020), and RDA analysis (packages ‘vegan’ and ‘Hmisc’—Harrell Jr 2022).
Results
Water chemistry and other parameters
Sampling sites did not vary greatly in many parameters that had been estimated (Table 2). The salinity ranged within the boundaries for the freshwater lakes in the Antarctic (Laybourn-Parry and Wadham 2014). The concentration of dissolved oxygen was close to saturation and suprasaturation, which is also typical for Antarctic lakes and ponds with rich cyanobacterial flora (e.g.,Váczi and Barták 2011).
The values of some parameters are explained by the shallowness. The temperature reached high values due to rapid heating of the thin water layer by solar irradiance. Both small depth and stirring of the bottom determined the increase of the turbidity measured in NTU making some ponds to be considered as ‘much turbid’.
Species diversity
Bdelloid individuals were found in 26 of 28 (or 93%) sampled water bodies (Table 3). The single non-bdelloid rotifer, Lepadella patella (Müller, 1773) (Lepadellidae, Ploima), was recorded at one site, DC5, with abundance of about 70 ind m−3.
A total of nine bdelloid species including those that still have status of subspecies was recorded at the study area (γ diversity). More than half of the species belong to the genus Adineta (Adinetidae, Adinetida). Other species are members of the following genera: Habrotrocha (Habrotrochidae, Philodinida), Pleuretra, Philodina, Macrotrachela (Philodinidae, Philodinida). Two species (Adineta sp.1 and Habrotrocha sp. 1) possess new morphological features that do not match with any bdelloid species already described and hence can be considered as candidates for species new to science.
All species with notes on their taxonomy are listed below.
Phylum Rotifera Cuvier, 1798.
Class Eurotatoria De Ridder, 1957.
Subclass Bdelloidea Hudson, 1884.
Order Adinetida Melone and Ricci, 1995.
Family Adinetidae Hudson and Gosse, 1886.
Genus Adineta Hudson, 1886.
1. A. coatsi Iakovenko et al., 2015—Fig. 4a
Adineta coatsae Iakovenko et al. 2015: 11.
2. A. grandis Murray, 1910—Fig. 4b
A. grandis Murray, 1910: 51—Dartnall and Hollowday 1985: 33, Donner 1965: 273, Kutikova 2005: 275, Iakovenko et al. 2015: 17.
3. Adineta sp.1—Figs. 4c–e; 6a–c
Oviparous, moderate-sized (282–359 μm) rotifer with flattened body of yellow-orange color (Fig. 6a). Integument is smooth and transparent without any tubercles, humps, or granulation. BW/TL is 22–25% (Table 4). Head is oval, of moderate sizes, HL/TL is 18–19%, HW/HL is 78–90%. Five teeth in each rake. Despite the resemblance in exterior, the species is different from Adineta vaga (Davis, 1873) or Adineta emsliei Iakovenko et al. 2015 by the shape of rostrum lamella. The rostrum of Adineta sp.1 is long (length outside the edge of the head is 11 μm, which is 22% of the head length) and wide (19–20 μm) and is clearly visible while crawling (Fig. 6b). Two flat, not tubular-like, oval lobes (4–5 μm length) are bulged out the rostrum at both sides. No eyespots. The dental formula is 2/2. Neck and foot are of moderate length (NL/TL is 17–18% and FL/TL is 11–13%), the latter is quite clearly separated from the trunk. Foot and rump are wide: FW/FL is 47–65%; RW/RL is 82–112%. Spurs are short, triangular, with pointed tips, divided by interspace (Fig. 6c). Sometimes spurs are slightly curved. SL/SSW is 62–91%.
As only morphology was examined, more investigations are needed. Further studies using molecular analysis highly likely may resolve the rotifer as a new Antarctic endemic species.
4. Adineta cf. emsliei Iakovenko et al. 2015—Fig. 4f
Adineta emsliei Iakovenko et al. 2015: 16.
Oviparous, of moderate size (330–335 μm). The identification is questionable as both overall exterior and morphological details have differences with that of original description of A. emsliei and have similarities with some morphological forms of A. vaga species groupFootnote 1. First, short spurs are clear triangular not needle-shaped with bulb-like swollen bases. SL/SSW is 60% (Table 4), which is the lower limit for A. emsliei. Second, rakes have five teeth (rakes of A. emsliei have six teeth). Details of the rostrum (two flat, not tubular-like, triangular lobes with short cilia under them) are the same for both A. vaga and A. emsliei. Most of the proportions are within the ranges obtained for A. emsliei (Table 4, compare with Iakovenko et al. 2015). The head is rather big (HL/TL is close to upper limit for A. emsliei) that brings it closer to Adineta vaga major Bryce, 1893. By body size (TL) the species exceeds A. emsliei a little but is within the size range for A. vaga s.l. Masticatory apparatus larger in comparison with that of A. emsliei—17.1 μm and 15.7 μm, respectively. The body is colored, however, less than Antarctic endemic members of Adineta genus.
As only morphology was examined, more investigations are needed. Further studies using molecular analysis may correct the identification.
5. Adineta cf. vaga minor Bryce, 1893—Fig. 4g
Adineta vaga minor Bryce 1893: 146—Donner 1965: 274, Kutikova 2005: 275.
Very similar to the previous species by its general exterior, morphology details, and proportions (Table 4), body of moderate size (up to 350 μm). The identification was based on the fragile body, poorly visible segment boundaries, smooth transition between a trunk, a rump, and an elongated foot. Spurs are quite elongated, SL/SSW is up to 123%.
As only morphology was examined, more investigations are needed. Further studies using molecular analysis may correct the identification.
Order Philodinida Melone and Ricci, 1995.
Family Habrotrochidae Harring, 1913.
Genus Habrotrocha Bryce, 1910.
6. Habrotrocha sp. 1—Figs. 5a, b; 6d, e
The body is of moderate sizes (321–362 μm), slightly elongated, not massive, shaped like a spindle, with a foot being the narrowest part. BW/TL is 14–15% (Table 4). Integument is smooth and transparent without any tubercles, humps, or granulation. The body is yellow/bright orange-colored with well-visible lateral folds on the trunk during crawling. During feeding the most colored part is the trunk (stomach), while the head and the neck seem to be almost transparent. The rostrum is wide, rostrum lamella with two very small, barely visible lobes. The neck is of moderate length or short (NL/TL is 15–18%) and is of moderate width (MNW is 31–40 μm). Feeding individuals have very characteristic shapes: transparent head and neck, more massive and rectangular trunk with bright orange content of the stomach. Integuments form acuminate protrusions on the front corners of the trunk. Integument in the preanal segment creates strong thickening (Fig. 6d). Head possesses almost equal length and width (HW/HL is 97%). The corona is wider than the head, CW/HW is 111%. Pedicels have a semicircular shape from dorsal view, the sulcus is narrow. The upper lip is triangular with a rounded tip, goes up to slightly more than one-half of pedicels. Rump and foot are short and wide: RL/TL is 6–9%, RW/RL is 107–144%, FL/TL is 8–10%, FW/FL is 62–85%. Spurs are very short, triangular with rounded tips; diverge outward at an angle of 45º to the central body axis (Fig. 6e). SL/SSW is 48%. Food pellets are round and small (5 μm diameter). The dental formula is 4/3, 3/3, or 4/4, almost in equal proportions. No eyespots. Movements are not fast, individuals often feed.
Family Philodinidae Ehrenberg, 1838.
Genus Macrotrachela Milne, 1886.
7. Macrotrachela sp.
Genus Philodina.
8. P. gregaria Murray, 1910—Fig. 5c, d
P. gregaria Murray 1910: 42—Donner 1965: 202; Kutikova 2005: 221.
Genus Pleuretra Bryce, 1910.
9. Pleuretra lineata Donner, 1962—Fig. 5e
Pleuretra lineata Donner 1962: 324—Donner 1965: 189; Kutikova 2005: 197.
The observed γ diversity at the study area was significantly lower than the estimated number of the species, which constituted 23 (computed using Chao algorithm), 25 (ICE), and 15 (Jackknife estimation).
The observed number of species at site (α diversity) ranged from one to three. The mean (± SD) number of species per site was 1.7 ± 0.7 n = 28. Almost one-half of the sites harbored two species.
The differences between sites (β diversity) are hardly interpretable as very few species were recorded at more than one water body. The number of sites populated only by Philodinida members was higher than that of sites with only Adineta (six and four, respectively). None of the species was found at all sites. The most frequent was P. gregaria, which was registered at 15 sites, followed by A. grandis (eight sites) and A. coatsi (five sites). Six species were singletons (recorded in one water body). Sørensen distances across the pair-wise matrix range from 0 (fully overlap) to 1 (no same species in pair of sites). The species diversity between sampled localities (DC, BAS, etc.) varied to a greater extent compared with the diversity between sampled sites within one locality (ANOSIM: R = 0.36, p = 0.02, the number of permutations—9999). However, the geographic distance between sites appeared not to correlate the species composition in general (Mantel test: R = 0.041, p = 0.36, number of permutations—9999).
Ecology and distributional patterns
In total, Adineta species predominated over Philodinida species at 11 water bodies. The maximal abundance was recorded at UC1—770,000 ind∙m−3, wherein ≈746,900 ind∙m−3 were presented by Philodinida members (P. gregaria). At UC2, another pond from this locality, the total abundance of 139,000 ind∙m−3 was revealed, but A. grandis from Adineta genus constituted most of the individuals (130,660 ind∙m−3). Apart from these two species, in UC, the large abundance was also demonstrated by Habrotrocha sp. 1—5560 ind∙m−3.
UC locality, where we observed the highest bdelloid abundance, was followed by SM and DC localities, where bdelloids (all taxa) ranged 160–11,900 ind∙m−3 n = 2 and 28–5440 ind∙m−3 n = 5, respectively (Fig. 7a). However, Adineta members were less presented at these two localities, with only P. gregaria in all sites of DC, P. gregaria with P. lineata in similar proportions in one site of SM, and P. gregaria and Adineta sp.1 in another were found. In VM sites, the bdelloid abundances were from 0 to 4375 ind∙m−3 n = 6 and in BAS these values were from 0 to 587 ind∙m−3 n = 7 with Adineta predominating in both (Fig. 7a). Bdelloids at water bodies of other localities yielded less than 100 ind∙m−3(except of RH1 - 210 ind∙m−3).The statistical difference in abundances between different localities was revealed in the case of Adineta spp. (Kruskal–Wallis chi-squared test: H3 = 10.65, p = 0.01) and was not for Philodinida spp. and Bdelloidea in general.
Bdelloid abundances at a four sampling localities, water bodies with different b type of bottom or c extensivity of CBM, d different types of habitat. Some localities (Gnezdovoy Cape, Southern Mount, Northern Ridge, Rubin Hill) are not presented because of very limited dataset. Abundances of Adineta and Philodinida were not estimated in lakes as only quantitative samples had been collected. Boxes represent interquartile ranges, box central lines—median, whiskers—max and min, spots—outliers
Bdelloids were most abundant in rock-basin ponds (mean ≈300,000 ind∙m−3 n = 3; Fig. 7b), which include UC1 and UC2 sites, the most inhabited water bodies in the study. Along the different types of bottoms, bdelloid abundances were decreasing from a rock bottom in the following order: rock/ground—ground/soft sediments—ground. The latter was the most distributed bottom type (occurred in 15 water bodies), the abundances of bdelloids there ranging from very few to more than several thousands of ind∙m−3 (e.g., P. gregaria in DC1). The abundance of Adineta spp. was low in water bodies with ‘ground / soft sediments’ bottom while Philodinida spp., namely P. gregaria, were more numerous there (4 ind∙m−3 in one site and 1–4550 ind∙m−3 n = 4, respectively; Fig. 7b).
The sites, which demonstrated large bdelloid abundances (i.e., all DC and UC water bodies), were characterized by the extensive CBM. Sites with no or non-extensive CBM provide smaller values (0–11,900 ind∙m−3 n = 14 and 10–149 ind∙m−3 n = 3, respectively; Fig. 7c). In general, these tendencies remained for both large taxa considered. In some water bodies with extensive mats (UC2) Adineta spp. provided high values, whereas its mean value was much lower than that of Philodinida (≈19,250 ind∙m−3 n = 7 and ≈95,680 ind∙m−3 n = 8, respectively; Fig. 7c).
Nevertheless, no statistical differences in abundances were found in all cases of comparing between different bottom types and rates of the CBM extensity.
Bdelloid abundances in temporary water bodies were higher than in lakes (Fig. 7d), the difference was statistically highly significant (Mann–Whitney test: W = 109, p = 0.0006).
The RDA triplot (Fig. 8) was created focusing on response variables and their correlations (Scaling type II). Environmental data explain 78.4% of species variation. The first two axes explain 54.5% of the total variation and 69.6% of the constrained variation. The results of the RDA analysis of the rotifer community were statistically significant (permutation test: F13 = 1.67, p = 0.038).
RDA analysis of bdelloid species and tardigrades constrained by environmental parameters. Colored arrows represent response variables: Adineta species (red arrows), Philodinida species (green), tardigrades (blue). Black arrows represent water chemistry and other numerical explanatory variables (solid—for most important and dashed—for not significant). Black designations without arrows designate type of the bottom and CBM extensivity. Sites are shown as geometrical symbols: filled squares—BAS; diamonds—NR; filled circles—DC; downward facing triangles—SM; empty circles—UC; empty squares—VM; upward facing triangles—RH. Eight water bodies were excluded due to lack of environmental or species data. Agra A. grandis, Acoa A. coatsi, Asp1 Adineta sp. 1, Aems Adineta cf. emsliei, Avmi Adineta cf. vaga minor, Hsp1 Habrotrocha sp. 1, Msp Macrotrachela sp., Pgre P. gregaria, Plin Pleuretra lineata, nCBM no CBM, neCBM non-extensive CBM, eCBM extensive CBM
The strongest explanatory variables were altitude (explains 8.0% of variation; p = 0.02), TDS (6.6%; p = 0.029), salinity (6.5%; p = 0.028) and type of the bottom (9.9%; p = 0.043). The CBM extensity appeared not to influence the distribution of the bdelloid species (explains less variation than random normal variables), however, this factor is included in the consideration.
Bdelloidea species responded to environmental parameters differently. Adineta members, despite their very similar morphology of ‘scrapers’, did not show close tendencies in their distribution in general and explicitly were not linked with the extensive CBM as it was supposed. A. coatsi, A. cf. emsliei, and Adineta sp.1 were negatively correlated with extensive mats but were positively correlated with oxidation–reduction potential. Adineta cf. vaga minor was positively correlated with temperature and ground bottom. The most distributed species, A. grandis, was independent in relation to presence/absence of CBM but was better fitted to water bodies at low altitudes, with rock or ground/rock bottom, high TDS and salinity. Philodinida species also demonstrated variability in relation to environmental parameters. Habrotrocha sp. 1 was the only species linked with pH and responded to factors similarly to A. grandis: positively to rock bottom, TDS and salinity. Macrotrachela sp. was positively correlated to dissolved oxygen and ground/rock bottom. The only two species which were positively correlated with the extensive CBM were P. lineata, and—to a much greater extent—well-distributed P. gregaria. These species did not show links with the type of the bottom and coincided with tardigrades which were expectedly inclined to massive development of the cyanobacterial mats.
Discussion
The East Antarctica environment is characterized by extremely low temperatures, strong winds and deficiency of food resources and organic matter. The exception is large freshwater lakes, where liquid water provides a year-round thermal buffer against the climatic extremes (Gibson et al. 2006). Few studied water bodies match this characteristic, and the rest are likely to freeze completely, so organisms inhabiting these particular ecosystems must be able to somehow withstand temperatures significantly below 0 °C and total dryness in order to survive. As is known, the specific type of dormant condition, so-called ‘cryptobiosis’, which is a temporary cessation of any metabolic activity, ensures bdelloids’ survival for years in frozen or desiccated state (Crowe and Cooper 1971; Clegg 2001). The recent discovery of bdelloid rotifers being revived after retrieval from Siberian permafrost (Pleistocene Yedoma formation), radiocarbon-dated to ∼24,000 years BP (Shmakova et al. 2021) revealed that these organisms are one of the best examples in the world of adaptation to an extreme cold environment.
In samples collected from the Thala Hills, four endemic species were found. P. gregaria and A. grandisFootnote 2 were first described during pioneering studies of Antarctic invertebrates at lakes in the Cape Royds area (Murray 1910) and their large size and pigmentation are considered to be specific features of Antarctic bdelloids. These two species were plentiful in both Murray’s study and the one presented here. Other two species, A. emsliei and A. coatsi, were recently determined as a result of revising early Antarctic records and had previously been mentioned and depicted as A. vaga and Adineta barbata Janson, 1893, respectively (Iakovenko et al. 2015).
Two species, identified only to genus level, did not match any known taxon, which obviously offers the prospect of further studies focusing on taxonomy. Habrotrocha sp.1 and Adineta sp.1 may represent new species for Antarctica and for science as well.
A. vaga minor, which identification in the present study is questionable, is widely distributed taxon (Segers 2007), which early records in the Antarctic, as well as A. vaga early records, should be considered as A. emsliei (Iakovenko et al. 2015; Velasco-Castrillón et al. 2018). In the present study A. vaga minor distanced morphologically from the description of A. emsliei, and more resembles original description. Our surveys in the Thala Hills have already identified rotifers with almost the same morphology in limno-terrestrial systems (Lukashanets et al. 2019, 2021). However, as the molecular divergence between bdelloid populations across the world and Antarctic representatives is significant (Fontaneto et al. 2008, 2011; Iakovenko et al. 2015), aforementioned species may, in fact, be the species new for the science like Adineta sp. 1. Pleuretra lineata appears also to have a cosmopolitan distribution (Segers 2007; Iakovenko unpubl.), with proved records in the Maritime Antarctic (Iakovenko et al. 2015). Thereby, the Thala Hills oasis is the only region in East Antarctica where Pleuretra members have been recorded. The species record in aquatic ecosystem is no less interesting, since it was assumed that P. lineata is a ‘terrestrial’ rotifer, inhabiting wet mosses (Kutikova 2005).
Despite sufficient sample efforts and appropriate methods of identification, the observed species diversity is extremely low, with a significant proportion of singleton species recorded. The hypothetical number of species estimated using mathematical tools (Chao estimator, etc.) is more than twice as high. This can be explained by the recent origin of the Antarctic bdelloids, which is reflected in the low levels of genetic diversity of the species on the continent compared to other regions (Cakil et al. 2021). In general, the conception of extremely low levels of Antarctic diversity seems to be exemplified by our studies of bdelloid rotifers, albeit it must be verified by studying microscopic animals in surrounding mosses, soil, and other habitats.
That species distances do not correlate with geographic distances between sites (proved by the Mantel test) points to the significant dispersal capacities of micro-invertebrates enhanced by drivers specific for the Antarctic. Compared to other zooplankton organisms, bdelloid rotifers seem to demonstrate the strongest rates of colonization by means of wind and rain (Caceres and Soluk 2002). Fontaneto and Ricci (2006) and Debortoli et al. (2018) also have claimed that bdelloid propagules (‘tuns’—contracted bodies after entering the specific dormant stage) can easily be transported by air currents. In Continental Antarctica, bdelloids are highly likely to be spread by extreme winds (up to 50 m s−1 in the region of the Thala Hills) and blizzards. Liquid water, which is usually considered to be the main driver of species proliferation in the Antarctic (Block et al. 2009; Convey et al. 2014), cannot cause massive transportation between sites because they are isolated from one another, with solid ice and rocks in between. Moreover, the period of melting is extremely short and lasts from the middle of December to early February. Birds, the third important vector of aquatic invertebrate dispersal (Moreno et al. 2019), hardly function in this way in Continental Antarctica, as the only flying species common for the area is a south polar skua (Lukashanets et al. 2021), which is neither abundant nor associated with inland waters.
However, even though species distances between sites do not correlate with geographic distances, it was shown that species distances within one locality are lower than species distances between localities (ANOSIM); i.e., the broad-scale diversity does not match the local diversity. This means that the species distribution is not fully randomized (a so-called ‘randomized immigration pattern’, according to Finlay 2002), and dispersal is still partially limited by certain barriers.
Altitude is one of the most important factors determining the bdelloid distribution, especially A. grandis, the most common species. Relating to why lowland water bodies close to the coast are more enriched with bdelloids we can only make some speculations. For example, high-altitude sites, located towards the ice sheet can be impoverished by downward katabatic winds have been flowing out the substrates, along with organisms themselves. Among water parameters, TDS and salinity appeared to be the most crucial having both a strong influence on A. grandis distribution. NB A. coatsi, another member of the Adineta genus, following A. grandis in terms of occurrence frequency, is not influenced by these parameters. Some species belonging to both Adineta and Philodinida are fitted to particular types of the bottom substrate. Considering that the distribution of Antarctic micrometazoans is patchy (Adams 2006; Heatwole and Miller 2019), associations between types of bottoms and species look like a ‘by-chance picture’, without a biological or ecological basis. Such strong, but hard-to-explain biases for several species led to the significant correlation of the ‘bottom’ factor with the entire variation.
Microbial bottom mats, which are widely distributed in polar areas, strongly influence biotic interactions in small lakes and ponds, providing the bulk of the autotrophic production (Rautio et al. 2011) and nutritional basis for aquatic animals (Kleinteich 2013). The latter can probably live in these three-dimensional and complex formations, or at least graze on them. For example, the metabarcoding analysis showed that CBM are refugia for Arctic eukaryotes (Jungblut et al. 2012). In the Antarctic, microbial mats supply the nutrients for scarce and seasonal plankton (Gibson et al. 2006), and provide the habitats for bdelloids, tardigrades, and nematodes (Wada et al. 2022). Some studies also indicated the extremely high numbers of bdelloids in CBM-like, but terrestrial habitats—alga Prasiola crispa growing on the ground or rock (Lukashanets et al. 2022).
All the foregoing made us first suppose that CBM are an important characteristic of the water body, shaping the rotifer community there. However, the RDA proved the insignificance of the CBM in the variation of the bdelloid distribution. Ironically, a positive correlation with mats was found in the case of the most distributed Philodinida rotifer—P. gregaria, which is an opportunistically planktonic species and alternates between attaching itself to substrata and swimming and feeding. This species seems to benefit in the bottom microbe- and nutrient-rich environment and coexists with tardigrades, which expectedly emerge in the water because of disturbance of CBM. In contrast, the numbers of crawling scrapers from the Adineta genus were not correlated or even correlated negatively to the spatial development of CBM, which in fact should specifically be considered. The phenomenon whereby barely planktonic organisms inhabit water bodies (in vast numbers) as well as potential pathways of their emerging should be explored to shed light on hazy processes of the formation of ecosystems in Antarctic oases. Before that only general assumptions can be made. For example, bdelloids can be associated with minute ground particles or debris either raised from the bottom or delivered from the surroundings. Or, as a significant component of the glacial biota (Zawierucha et al. 2020), they can settle in temporal water bodies during short austral summers from snow or ice, simply from processes associated with freezing-melting seasonal dynamics.
Data availability
All data which are mandatory for demonstrating the results of the study are included in this published article. The primary data (e.g., measurements in water bodies) are available from the corresponding author on request.
Notes
References
Adams BJ, Bardgett RD, Ayres E, Wall DH, Aislabie J, Bamforth S, Bargagli R, Cary C, Cavacini P, Connell L, Convey P, Fell JW, Frati F, Hogg ID, Newsham KK, O’Donnell A, Russell N, Seppelt RD, Stevens MI (2006) Diversity and distribution of Victoria land biota. Soil Biol Biochem 38:3003–3018
Andrews WA (1972) A guide to the study of freshwater ecology. Prentice Hall, NJ
Bégin PN, Vincent WF (2017) Permafrost thaw lakes and ponds as habitats for abundant rotifer populations. Arct Sci 3(2):354–377. https://doi.org/10.1139/as-2016-0017
Block W, Lewis Smith RI, Kennedy AD (2009) Strategies of survival and resource exploitation in the Antarctic fellfield ecosystem. Biol 84:449–484
Caceres CE, Soluk DA (2002) Blowing in the wind: a field test of overland dispersal and colonization by aquatic invertebrates. Oecologia 131:402–408. https://doi.org/10.1007/s00442-002-0897-5
Cakil Z, Garlasché G, Iakovenko N, di Cesare A, Eckert E, Guidetti R, Hamdan L, Janko K, Lukashanets D, Rebecchi L, Schiaparelli S, Sforzi T, Kašparová E, Velasco-Castrillón A, Walsh E, Fontaneto D (2021) Comparative phylogeography reveals consistently shallow genetic diversity in a mitochondrial marker in Antarctic bdelloid rotifers. J Biogeogr 48:1797–1809. https://doi.org/10.1111/jbi.14116
Camacho A (2006) Planktonic microbial assemblages and the potential effects of metazooplankton predation on the food web of lakes from the maritime Antarctica and sub-Antarctic islands. Rev Environ Sci Biotechnol 5:167–185. https://doi.org/10.1007/s11157-006-0003-2
Camacho A, Rochera C, Villaescusa JA, Velázquez D, Toro M, Rico E, Fernandez-Valiente E, Justel A, Banon M, Quesada A (2012) Maritime Antarctic lakes as sentinels of climate change. Int J of Design & Nature and Ecodynamics 7(3):239–250
Chown SL, Lee JE, Hughes KA, Barnes J, Barrett P, Bergstrom D, Convey P, Cowan D, Crosbie K, Dyer G, Frenot Y, Grant S, Herr D, Kennicutt M, Lamers M, Murray A, Possingham H, Reid K, Riddle M, Wall D (2012) Challenges to the future conservation of the Antarctic. Science 337:158–159
Clegg JS (2001) Cryptobiosis – a peculiar state of biological organization. Comp Biochem Physiol B 128(4):613–624. https://doi.org/10.1016/s1096-4959(01)00300-1
Conservation of Antarctic Fauna and Flora (2009) Annex II to the Protocol on Environmental Protection to the Antarctic Treaty. Measure 16 Attachment. Conservation of Antarctic Fauna and Flora, Brussels
Convey P (2010) Terrestrial biodiversity in Antarctica – recent advantages and future challenges. Polar Sci 4:135–147
Convey P, Peck LS (2019) Antarctic environmental change and biological responses. Sci Adv 5:11
Convey P, Stevens MI (2007) Antarctic biodiversity. Science 317:1877–1878
Convey P, Chown SL, Clarke A, Barnes DKA, Cummings V, Ducklow H, Frati F, Green TGA, Gordon S, Griffiths H, Howard-Williams C, Huiskes AHL, Laybourn-Parry J, Lyons B, McMinn A, Peck LS, Quesada A, Schiaparelli S, Wall D (2014) The spatial structure of Antarctic biodiversity. Ecol Monogr 84:203–244
Crowe JH, Cooper AF Jr (1971) Cryptobiosis Sciam 225(6):30–36
Dartnall HJG (1995) Rotifers, and other aquatic invertebrates, from the Larsemann hills, Antarctica. Pap Proc R Soc Tasmania 129:17–23
Dartnall HJG (2000) A limnological reconnaissance of the Vestfold Hills. Australian Antarctic Division, Kingston
Dartnall HJG (2017) The freshwater fauna of the south polar region: a 140-year review. Pap Proc R Soc Tasmania 151:19–58
Dartnall HJG, Hollowday ED (1985) Antarctic rotifers. Sci Rep Br Antarct Surv 100:1–46
Debortoli N, de Laender F, Doninck K (2018) Immigration from the metacommunity affects bdelloid rotifer community dynamics most. BioRxiv. https://doi.org/10.1101/450627
Díaz A, Maturana CS, Boyero L, de LosEscalante PR, Tonin AM, Correa-Araneda F (2019) Spatial distribution of freshwater crustaceans in Antarctic and Subantarctic lakes. Sci Rep. https://doi.org/10.1038/s41598-019-44290-4
Donner J (1965) Order Bdelloidea (Rotatoria, Rotifers). Identification guides of the soil fauna of Europe. Akademie, Berlin 37:406–412 (in German)
Ellis-Evans JC (1996) Microbial diversity and function in Antarctic freshwater ecosystems. Biodivers Conserv 5:1395–1431. https://doi.org/10.1007/BF00051985
Finlay BJ (2002) Global dispersal of free-living microbial eukaryote species. Science 296(5570):1061–1063
Fontaneto D, Ricci C (2006) Spatial gradients in species diversity of microscopic animals: the case of bdelloid rotifers at high altitude. J Biogeogr 33(7):1305–1313
Fontaneto D, Barraclough TG, Chen K, Ricci C, Herniou EA (2008) Molecular evidence for broad-scale distributions in bdelloid rotifers: everything is not everywhere but most things are very widespread. Mol 17(13):3136–3146. https://doi.org/10.1111/j.1365-294X.2008.03806.x
Fontaneto D, Iakovenko N, Eyres I, Kaya M, Wyman M, Barraclough TG (2011) Cryptic diversity in the genus Adineta Hudson & Gosse, 1886 (Rotifera: Bdelloidea: Adinetidae): a DNA taxonomy approach. Hydrobiologia 662:27–33. https://doi.org/10.1007/s10750-010-0481-7
Fontaneto D, Iakovenko N, De Smet W (2015) Diversity gradients of rotifer species richness in Antarctica. Hydrobiologia 761(1):235–248. https://doi.org/10.1007/s10750-015-2258-5
Gibson JAE, Wilmotte A, Taton A, van de Vijver B, Beyens L, Dartnall HJG (2006) Biogeographic trends in Antarctic lake communities. In: Bergstrom DM, Convey P, Huiskes AHL (eds) Trends in Antarctic Terrestrial and Limnetic Ecosystems. Springer, Dordrecht, pp 71–99
Gotelli NJ, Colwell RK (2011) Estimating species richness. In: Magurran AE, McGill BJ (eds) Biological Diversity: Frontiers in Measurement and Assessment. Oxford University Press, Oxford, pp 39–54
Hansson LA, Hylander S, Dartnall HJG, Lidström S, Svensson JE (2012) High zooplankton diversity in the extreme environments of the McMurdo dry valley lakes, Antarctica. Antarct Sci 24:131–138. https://doi.org/10.1017/S095410201100071X
Harrell Jr FE (2022) Package ‘Hmisc’ (Version 4.7–1). https://cran.r-project.org/web/packages/Hmisc/Hmisc.pdf. Accessed 27 Oct 2022
Heatwole H, Miller WR (2019) Structure of micrometazoan assemblages in the Larsemann Hills, Antarctica. Polar Biol 42:1837–1848
Hijmans RJ (2021) Introduction to the "geosphere" package (Version 1.5–14). https://cran.r-project.org/web/packages/geosphere/vignettes/geosphere.pdf. Accessed 27 Oct 2022
Iakovenko NS, Kašparová E, Plewka M, Janko K (2013) Otostephanos (Rotifera, Bdelloidea, Habrotrochidae) with the description of two new species. Syst Biodivers 24:1–13. https://doi.org/10.1080/14772000.2013.857737
Iakovenko NS, Smykla J, Convey P, Kašparová E, Kozeretska IA, Trokhymets V, Dykyy I, Plewka M, Devetter M, Duris Z, Janko K (2015) Antarctic bdelloid rotifers: diversity, endemism and evolution. Hydrobiologia 761:5–43. https://doi.org/10.1007/s10750-015-2463-2
Izaguirre I, Allende L, Romina Schiaffino M (2021) Phytoplankton in Antarctic lakes: biodiversity and main ecological features. Hydrobiologia 848:177–207. https://doi.org/10.1007/s10750-020-04306-x
Jungblut AD, Vincent WF, Lovejoy C (2012) Eukaryotes in Arctic and Antarctic cyanobacterial mats. FEMS Microbiol 82(2):416–428. https://doi.org/10.1111/j.1574-6941.2012.01418.x
Kleinteich J (2013) Diversity and ecophysiology of cyanobacterial mat communities in Arctic and Antarctic ecosystems. Dissertation, Konstanz Universität
Koste W (1996) To the fauna of moss rotifers in Madagascar. Osnabrücker Naturwissenschaftliche Mitteilungen 22:235–253 (in German)
Kutikova LA (1991) Rotifers of the inland waters of East Antarctica. Sov Ant Exped Inf Bul 116:87–99 (in Russian)
Kutikova LA (2005) Bdelloid rotifers in the fauna of Russia. The community of scientific publications KMK, Moscow (in Russian)
Laybourn-Parry J, Pearce DA (2007) The biodiversity and ecology of Antarctic lakes: models for evolution. Phil Trans R Soc B 362:273–289. https://doi.org/10.1098/rstb.2006.1945
Laybourn-Parry J, Wadham J (2014) Antarctic Lakes. Oxford University Press, Oxford
Laybourn-Parry J, Ellis-Evans JC, Butler H (1996) Microbial dynamics during the summer ice-loss phase in maritime Antarctic lakes. J Plankton Res 18(4):495–511. https://doi.org/10.1093/plankt/18.4.495
Laybourn-Parry J, James M, McKnight DM, Priscu J, Spaulding SA, Shiel R (1997) The microbial plankton of lake Fryxell, southern Victoria land, Antarctica during the summers of 1992 and 1994. Polar Biol 17:54–61. https://doi.org/10.1007/s003000050104
Lukashanets D, Vezhnavets VV, Maysak NN, Hihiniak YH, Borodin OI, Miamin VE, Gaidashov AA, Nikitiuk LA (2019) Rotifers (Rotifera) from the inland waters and terrestrial habitats of East Antarctic oases (Enderby Land and Prydz Bay). Fragm Faunist 62(2):67–86. https://doi.org/10.3161/00159301FF2019.62.2.067
Lukashanets DA, Convey P, Borodin OI, Miamin VY, Hihiniak YH, Gaydashov AA, Yatsyna AP, Vezhnavets VV, Maysak NN, Shendrik TV (2021) Eukarya biodiversity in the Thala hills. East Antarctica Antarct Sci 33(6):605–623. https://doi.org/10.1017/S0954102021000328
Lukashanets DA, Miamin VE, Hihinyak YG (2022) The phenomenon of extremely high abundances of the Prasiola crispa-associated micrometazoans in East Antarctica. Polar Res 47:1–15. https://doi.org/10.33265/polar.v41.7781
Moreno E, Pérez-Martínez C, Conde-Porcuna JM (2019) Dispersal of rotifers and cladocerans by waterbirds: seasonal changes and hatching success. Hydrobiologia 834:145–162. https://doi.org/10.1007/s10750-019-3919-6
Murray J (1910) Biology. British Antarctic expedition 1907–1909, under the command of Sir E.H. Shackleton Rep Sci Investig 1:1–105
Oksanen J, Blanchet JG, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O'Hara RB, Simpson GL, Solymos P, Stevens MHM, Szoecs E, Wagner H (2020) vegan: Community ecology package. R package version 2.5–7. https://CRAN.R-project.org/package=vegan. Accessed 27 Oct 2022
Örstan A (2020) The trouble with Adineta vaga (Davis, 1873): a common rotifer that cannot be identified (Rotifera: Bdelloidea: Adinetidae). Zootaxa 4830(3):597–600. https://doi.org/10.11646/zootaxa.4830.3.8
Paliy O, Shankar V (2016) Application of multivariate statistical techniques in microbial ecology. Mol 25(5):1032–1057. https://doi.org/10.1111/mec.13536
R Core Team (2021) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL https://www.R-project.org/ Accessed 15 January 2022
Rautio M, Dufresne F, Laurion I, Bonilla S, Vincent WF, Christoffersen KS (2011) Shallow freshwater ecosystems of the circumpolar Arctic. Ecoscience 18:204–222. https://doi.org/10.2980/18-3-3463
Rochera C, Camacho A (2019) Limnology and aquatic microbial ecology of Byers Peninsula: a main freshwater biodiversity hotspot in maritime Antarctica. Diversity 11:201. https://doi.org/10.3390/d11100201
Rogers AD, Johnston NM, Murphy EJ, Clarke A (2012) Antarctic ecosystems: an extreme environment in a changing world. Wiley-Blackwell, Oxford
SCAR’s Environmental Code of Conduct for Terrestrial Scientific Field Research in Antarctica (2018) Resolution 5 Annex to ATCM XLI Final Report. Scientific Committee on Antarctic Research, Cambridge
Schwoerbel J (1972) Methods of hydrobiology. 1st edition. Freshwater ecology. Pergamon Press, Oxford
Segers H (2007) Annotated checklist of the rotifers (Phylum Rotifera), with notes on nomenclature, taxonomy and distribution. Zootaxa 1564(1–104):1–104. https://doi.org/10.11646/zootaxa.1564.1.1
Shmakova L, Malavin S, Iakovenko N, Vishnivetskaya T, Shain D, Plewka M, Rivkina E (2021) A living bdelloid rotifer from 24,000-year-old Arctic permafrost. Curr Biol 31(11):712–713. https://doi.org/10.1016/j.cub.2021.04.077
Simmons GM, Vestal JR, Wharton RA (1993) Environmental regulators of microbial activity in continental Antarctic lakes. In: Friedmann EI (ed) Antarctic microbiology. Wiley-Liss, New York, pp 491–541
Sudzuki M (1964) On the microfauna of the Antarctic region. I. Moss–water community at Langhovde. JARE 1956–1962 Sci Rep Series E 19:41
Váczi P, Barták M (2011) Summer season variability of dissolved oxygen concentration in Antarctic lakes rich in cyanobaterial mats. Czech Polar Rep 1:42–48
Velasco-Castrillón A, Hawes I, Stevens M (2018) 100 years on: a re-evaluation of the first discovery of microfauna from ross island. Antarctica Antarct Sci 30(4):209–219. https://doi.org/10.1017/S095410201800007X
Vincent WF (1988) Microbial ecosystems in Antarctica. Cambridge University Press, Cambridge
Vincent WF (2000) Evolutionary origins of Antarctic microbiota: invasion, selection and endemism. Antarct Sci 12:374–385
Vyverman W, Verleyen E, Wilmotte A, Hodgson D, Willems A, Peeters K, van de Vijver B, de Wever A et al (2010) Evidence for widespread endemism among Antarctic micro-organisms. Polar Sci 4:103–113
Wada T, Kudoh S, Koyama H, Iakovenko N, Elster J, Kvíderová J et al (2022) Assemblage of bdelloid rotifers in the microbial mats from East Antarctica: The ecological interactions between microscopic phototrophs and invertebrates. In: Špoljar M et al (eds) Book of abstracts of XIV International Rotifera Symposium. University of Zagreb, Zagreb, p 136
Zawierucha K, Porazinska DL, Ficetola GF, Ambrosini R, Baccolo G, Buda J, Ceballos JL, Devetter M, Dial R, Franzetti A, Fuglewicz U, Gielly L, Łokas E, Janko K, Novotna Jaromerska T, Kościński A, Kozłowska A, Ono M, Parnikoza I, Pittino F, Poniecka E, Sommers P, Schmidt SK, Shain D, Sikorska S, Uetake J, Takeuchi N (2020) A hole in the nematosphere: tardigrades and rotifers dominate the cryoconite hole environment, whereas nematodes are missing. J Zool 313(1):18–36. https://doi.org/10.1111/jzo.12832
Zuur A, Ieno E, Smith G (2007) Analysing ecological data. Springer Science + Business Media, New York
Acknowledgements
Authors would like to thank the members of the 11th Belarus Antarctic Expedition: surgery doctor Sergey Sukharev, engineer Alexey Hatkevich and physician Vladislav Bazylevich for delivery the samples from the Antarctic; chief of the expedition Alexey Gaidashov for assistance in the field and technical support. We are grateful to ResearchGate users Haitao Wang, Felipe Rego, and one person remained anonymous through account had been deleted for their discussions on performing the statistical tests. We wish to thank Dr. Diego Fontaneto for constructive reviewing of the manuscript, and Dr. Nataliia Iakovenko for reviewing and very valuable comments on taxonomy of bdelloids. Remarks given by Dieter Piepenburg, Journal’s editor in chief, significantly improved the article.
Author information
Authors and Affiliations
Contributions
DL designed the study, performed fieldwork and identification of bdelloid rotifers, carried out all statistical analyses, prepared the manuscript. NM analyzed rotifer abundances in samples.
Corresponding author
Ethics declarations
Conflict of interest
The authors report no conflict of interest.
Ethical approval
All sampling activities strictly followed the guidance of Annex II to the Protocol on Environmental Protection to the Antarctic Treaty (Conservation of Antarctic Fauna and Flora 2009) and the guidelines of the Scientific Committee on Antarctic Research (SCAR’s Environmental Code of Conduct for Terrestrial Scientific Field Research in Antarctica 2018).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lukashanets, D.A., Maisak, N.N. Bdelloid rotifers (Bdelloidea, Rotifera) in shallow freshwater ecosystems of Thala Hills, East Antarctica. Polar Biol 46, 87–102 (2023). https://doi.org/10.1007/s00300-022-03106-4
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00300-022-03106-4