Summary
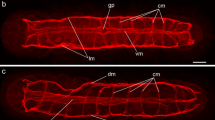
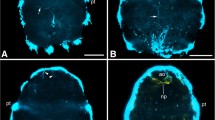
The nervous system (NS) of Microstomum lineare (Turbellaria, Macrostomida) was studied by electron and light microscopy, combined with fluorescence histochemistry (Falck-Hillarp method for biogenic monoamines). The NS is primitively organized, with a bilobed brain, two lateral nerve cords lacking commissures, and peripheral nerve cells scattered along the nerve cords. The stomatogastric NS, with a pharyngeal nerve ring, is joined to the central NS by a pair of connective ganglia. A green fluorescence in all parts of the NS indicates catecholaminergic neurons as the dominant neuron type.
Ultrastructurally, two types of neurons were identified on the basis of their vesicle content: 1. Aminergic (catecholaminergic) neurons containing densecore vesicles of varying electron-density and size, i.e., small dense-core vesicles (diameter 50–100 nm), vesicles with a highly electron-dense core (60–140 nm), and vesicles with an eccentric dense-core. 2. Presumed peptidergic neuro-secretory neurons containing large granular vesicles (diameter about 200 nm) in the stomatogastric NS and peripheral parts of the central NS. In light microscopy, paraldehyde-thionin stained neurons were observed in the same areas.
Similar content being viewed by others
References
Bullock TH, Horridge GA (1965) Structure and Function in the Nervous System of Invertebrates. IWH Freeman, San Francisco, Vol 1
Dahl E, Falck B, Mecklenburg C von, Myhrberg H (1963) Adrenergic sensory neurons in invertebrates. Gen Comp Endocrinol 3:693
Falck B, Owman Ch (1965) A detailed methodological description of the fluorescence method for the cellular demonstration of biogenic monoamines. Acta Univ Lund Sect II 7:1–23
Falck B, Hillarp N-Å, Thieme G, Torp A (1962) Fluorescence of catecholamines and related compounds condensed with formaldehyde. J Histochem 10:348–354
Gabe M (1966) Neurosecretion. Pergamon Press, Oxford
Gersch M (1975) Prinzipien neurohormonaler und neurohumoraler Steuerung physiologischer Prozesse. Friedrich-Schiller-Universität Jena, 151p
Grasso M (1975) Sexuality and neurosecretion in freshwater planarians. In: Reinboth R (ed) Intersexuality in the Animal Kingdom. Springer Verlag, Berlin, Heidelberg, New York, pp 20–29
Grasso M, Quaglia A (1970a) Studies on neurosecretion in planarians. I. Neurosecretory fibres near the testes of Dugesia lugubris. J Submicrosc Cytol 2:119–125
Grasso M, Quaglia A (1970b) Studies on neurosecretion in planarians. II. Observations on the ovaries of Dugesia lugubris. J. Submicrosc Cytol 2:127–132
Grasso M, Quaglia A (1971) Studies on neurosecretion in planarians. III. Neurosecretory fibres near the testes and ovaries of Polycelis nigra. J Submicrosc Cytol 3:171–180
Grasso M, Montanaro L, Quaglia A (1975) Studies on the role of neurosecretion in the induction of sexuality in a planarian agamic strain. J Ultrastruct Res 52:404–408
Hyman LH (1951) Platyhelminthes and Rhyncocoela. In: The Invertebrates. McGraw-Hill, New York, Vol. 2
Kerkut GA (1973) Catecholamines in invertebrates. Br Med Bull 29:100–104
Koopowitz H, Chien P (1974) Ultrastructure of the nerve plexus in flatworms. I. Peripheral organization. Cell Tissue Res 155:337–351
Lender T (1974) The role of neurosecretion in freshwater planarians. In: Reiser NW, Morese HP (eds) Biology of the Turbellaria, Libbie H Hyman memorial volume. McGraw-Hill, New York, pp 460–476
Lentz TL (1967) Fine structure of nerve cells in a planarian. J Morphol 121:323–338
Lentz TL (1968) Primitive Nervous Systems. Yale University Press, New Haven, Connecticut
Luft JH (1961) Improvements in epoxy embedding techniques. J Biophys Biochem Cytol 9:409–414
Lur'e BL (1975) Monoamine-containing neurons in the planarian Polycelis nigra (Turb) Vestn Mosk Univ Ser VI Biol Pochoved 30:3–13
Moraczewski J, Czubaj A, Bakowska J (1977a) Organization and ultrastructure of the nervous system in Catenulida (Turbellaria). Zoomorphologie 87:87–95
Moraczewski J, Czubaj A, Kwiatkowska J (1977b) Localization of neurosecretion in the nervous system of Catenulida (Turbellaria). Zoomorphologie 87:97–102
Morita M, Best JB (1965) Electron microscopic studies on planaria. II. Fine structure of the neurosecretory system in the planarian Dugesia dorotocephala. J. Ultrastruct Res 13:396–408
Morita M, Best JB (1966) Electron microscopic studies of planaria. III. Some observations on the fine structure of planarian nervous tissue. J Exp Zool 161:391–412
Oosaki T, Ishii S (1965) Observations on the ultrastructure of nerve cells in the brain of the planarian Dugesia gonocephala. Z Zellforsch 66:782–793
Paget GS (1959) Aldehyde-thionin: a stain having similar properties to aldehyde-fuchsin. Stain Technol 34:223–226
Palmberg I, Reuter M, Wikgren M (1980) Ultrastructure of the epidermal eyespots of Microstomum lineare. (Turbellaria, Macrostomida). Cell Tissue Res (in press)
Reisinger E (1976) Zur Evolution des stomatogastrischen Nervensystems bei den Plathelminthen. Z Zool Syst Evolutionsforsch 14:241–253
Reuter M, Lindroos P (1979) The ultrastructure of the nervous system of Gyratrix hermaphroditus (Turbellaria, Rhabdocoela). I. The brain. Acta Zool (Stockh) 60:139–152
Sauzin-Monnot MJ (1972) Etude ultrastructurale du tissue nerveux et des produits de sécrétion nerveuse, au cours des premières heures de régéneration de la planarie Polycelis nigra (Turbellarie-Triclade) au niveau de la blessure. Ann Embryol Morphol 5:257–265
Scharrer B (1978) Current concepts on the evolution of the neurosecretory neuron. In: Bargmann W, Oksche A, Polenov A, Scharrer B (eds) Neurosecretion and Neuroendocrine Activity. Evolution, Structure and Function. Proc VII th International Symposium on Neurosecretion. Leningrad, Aug 15–21, Springer-Verlag, Berlin, Heidelberg
Steel CGH, Morris GP (1977) A simple technique for selective staining of neurosecretory products in epoxy sections with paraldehyde fuchsin. Can J Zool 55:1571–1575
Welsh JM, Moorhead M (1960) The quantitative distribution of 5-hydroxytryptamine in the invertebrates, especially in their nervous system. J Neurochem 6:146–169
Welsh JH, King EC (1970) Catecholamines in planarians. Comp Biochem Physiol 36:683–688
Welsh JH, Williams JD (1970) Monoamine-containing neurons in Planaria. J Comp Neurol 138:103–116
Author information
Authors and Affiliations
Rights and permissions
About this article
Cite this article
Reuter, M., Wikgren, M. & Palmberg, I. The nervous system of Microstomum lineare (Turbellaria, Macrostomida). Cell Tissue Res. 211, 31–40 (1980). https://doi.org/10.1007/BF00233720
Accepted:
Issue Date:
DOI: https://doi.org/10.1007/BF00233720