- India
- International
The science behind a nuclear bomb
As Christopher Nolan’s Oppenheimer ignites conversation around nuclear weapons, a look at how they actually work.
 Castle Romeo nuclear test (yield 11 Mt) on Bikini Atoll by the US on March 27, 1954.
Castle Romeo nuclear test (yield 11 Mt) on Bikini Atoll by the US on March 27, 1954. There is a lot of chatter around nuclear weapons currently. President Vladimir Putin has suggested that if pushed to the wall, he would not be stopped from using them against Ukraine (which would almost certainly invite catastrophic NATO retaliation). At the G7 meeting in Hiroshima, Japan, the only country to have suffered a nuclear strike, pushed for a world without nuclear weapons.
And over the last three weeks, Christopher Nolan’s blockbuster ‘Oppenheimer’ has ignited conversation on the history of creation of these weapons, and the ethical dilemmas involved in their use.
But what is a nuclear weapon, to begin with? What is the science of the bomb, and from where does it get its terrifying power? What engineering and scientific capabilities — and natural resources — must be harnessed to create this modern miracle of science?
First, a primer on the atom.
Atoms are the basic building blocks of all matter, such that they cannot be “broken down” further by simple chemical processes. From our bodies to the air we breathe, everything is made up of atoms.
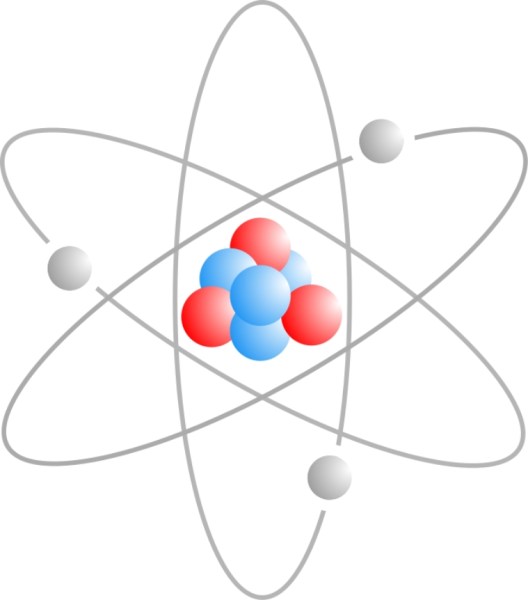 Atomic structure of Lithium-7 which has 3 protons, 4 neutrons and 3 electrons. (Wikimedia Commons)
Atomic structure of Lithium-7 which has 3 protons, 4 neutrons and 3 electrons. (Wikimedia Commons)
Most of an atom is empty space. The rest comprises three basic types of subatomic (smaller than, or occurring within an atom) particles — positively charged protons, negatively charged electrons, and the neutral neutrons. The protons and neutrons combine to form the atom’s nucleus, around which circle a “cloud” of electrons.

The number of protons in an atom determines the element, and the number of neutrons determines the isotope of that element. Different isotopes of the same element have the same chemical properties, but very different nuclear properties.
Let us now talk about nuclear fission, U-235, and chain reaction.
Most atoms on Earth are stable due to an equilibrated composition of neutrons and protons in their nucleus. However, in some unstable atoms, the composition of the number of protons and neutrons is such that it does not allow the nucleus to hold itself together.
Such atoms are known to be radioactive, and they tend to break apart or fission into two lighter elements. This is the basis of most nuclear weapons and atomic energy.
Uranium-235, an extremely rare isotope of the heavy metal uranium, is the most commonly used nuclear fuel, as it is one of the few elements that can undergo induced fission. This means that the element can be broken down very quickly by a process put into motion by humans.
This is done by subjecting a U-235 nucleus to neutrons. The nucleus immediately absorbs an extra neutron and consequently becomes unstable — and immediately breaks apart into two lighter atoms, and a few extra neutrons. This process releases what is known as atomic energy.
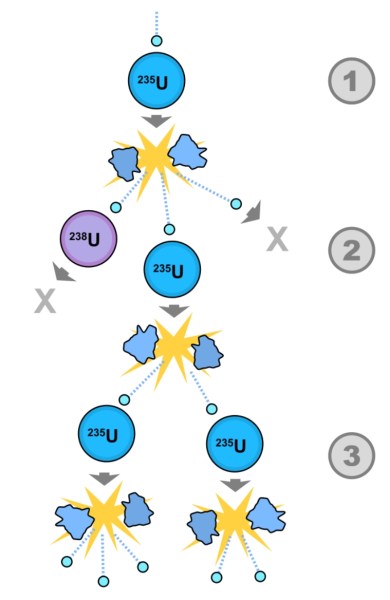 Schematic diagram of a fission chain reaction. A U-235 atom absorbs a neutron, and fissions into two new atoms (fission fragments), releasing three new neutrons and some binding energy. Even if only one of these new neutrons collides with a U-235 atom (as shown in this graphic), which then fissions and releases two neutrons and some binding energy, the chain reaction continues. (Wikimedia Common)
Schematic diagram of a fission chain reaction. A U-235 atom absorbs a neutron, and fissions into two new atoms (fission fragments), releasing three new neutrons and some binding energy. Even if only one of these new neutrons collides with a U-235 atom (as shown in this graphic), which then fissions and releases two neutrons and some binding energy, the chain reaction continues. (Wikimedia Common)
The fission of a U-235 atom produces about 2 to 3 new neutrons on average. If these new neutrons are then absorbed by other U-235 atoms, it creates an exponentially growing chain reaction. The math is simple: with each ‘generation’ into the chain reaction, the number of atoms engaged can increase by 2 to 3 times.
Even though not all neutrons engage in the fission process, as long as each fission leads to more than one additional fission, the chain reaction grows exponentially and releases large amounts of energy.
Then comes the need to ‘enrich’ uranium.
All of the above seems simple enough in theory. When it comes to practical implementation, bomb designers have to solve a number of problems.
First there is the problem of the nuclear fuel itself. Approximately 99.3% of naturally occurring uranium is of the isotope U-238, which is not fissionable. Naturally occurring uranium, therefore, cannot be used in a weapon, or for that matter, in nuclear power plants.
 A series of gas centrifuges used to produce enriched uranium. This photo is of a US gas centrifuge plant in Piketon, Ohio in 1984. (Wikimedia Common)
A series of gas centrifuges used to produce enriched uranium. This photo is of a US gas centrifuge plant in Piketon, Ohio in 1984. (Wikimedia Common)
Thus, uranium ore is enriched in order to increase the concentration of U-235. Most nuclear power plants require an enrichment of 3-4% U-235 to sustain a chain reaction. Fission bombs on the other hand need closer to 90% enrichment.
This is done in specific enrichment facilities using some extremely complex equipment. Notably, the equipment needed to enrich fuel for nuclear power generations is the same as that needed to enrich it for a bomb — leading to one of the great challenges of enforcing nuclear non-proliferation.
Engineers need to figure out a way in which a nuclear weapon can be detonated.
Scientists need to make sure that the bomb does not reach critical mass earlier than intended — this is necessary to prevent an accidental explosion. Critical mass is the minimum mass of fissionable material required to sustain a nuclear fission.
Thus, in a fission bomb, the fuel is kept in separate subcritical masses and then brought together when the explosion is intended. Designers have come up with two principal types of trigger mechanisms to achieve this.
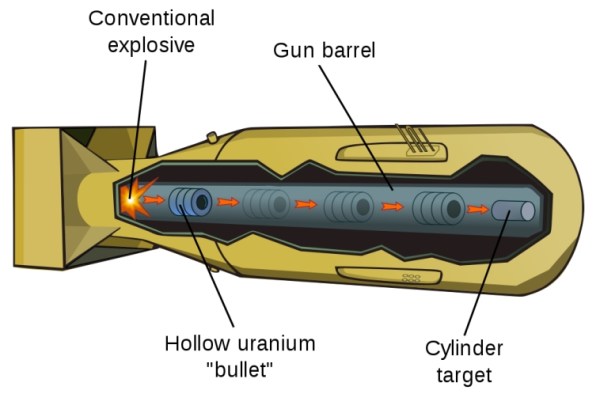 A visualisation of a U-235 fission bomb with a gun-type trigger mechanism. (Wikimedia Commons)
A visualisation of a U-235 fission bomb with a gun-type trigger mechanism. (Wikimedia Commons)
The simplest way to trigger the reaction is to make a “gun” fire one subcritical mass into another. One subcritical mass of U-235, shaped like a hollow cylinder “bullet”, is placed on one end of a barrel. The other is placed is placed on the other end as the “target”.
Conventional explosives shoot the bullet down the gun barrel, where it mates with the target, forming a supercritical mass, resulting in a chain reaction, and an atomic explosion. This was the kind of bomb that was used over Hiroshima.
There are also plutonium implosion devices.
The second way to create a supercritical mass is to compress the subcritical masses together by means of an implosion. This is the kind of device required for plutonium bombs, which cannot be triggered by a simple gun mechanism.
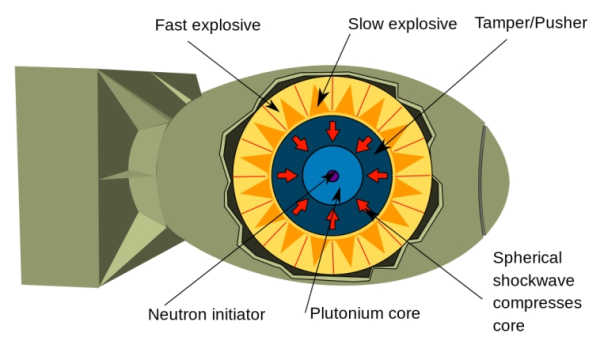 A plutonium implosion device is far more complex to build, but the required isotope of plutonium itself is easier to obtain in critical mass quantities. (Wikimedia Commons)
A plutonium implosion device is far more complex to build, but the required isotope of plutonium itself is easier to obtain in critical mass quantities. (Wikimedia Commons)
In 1941, scientists at the University of California at Berkeley discovered plutonium as a fissionable material. Plutonium implosion bombs are more complex to make but the fissionable isotope of plutonium, Pu-239, is easier and cheaper to obtain in critical mass quantities. If produced correctly, these bombs can also be more efficient than their U-235 counterparts.
The Trinity Test at Los Alamos and the bomb triggered over Nagasaki used plutonium as fuel.
Finally, there are the fusion bombs.
Fission bombs, although very destructive, are not very efficient. There is also a theoretical limit to the yield they can possibly generate. The largest fission bombs tested till date have had yields of around 500 kilotonnes.
Fusion bombs, on the other hand, have no such limit. The largest fusion bomb ever tested — the Soviet Tsar Bomba — gave a yield of roughly 50 megatonnes, or 50,000 kt.
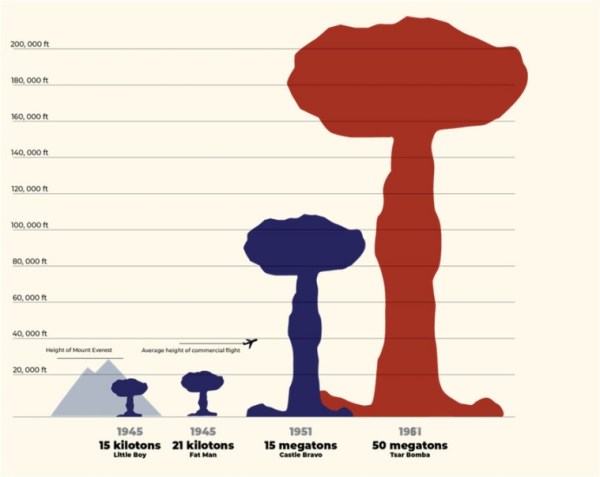 Nuclear Weapons Comparison. Source: Los Alamos National Laboratory (LANL)
Nuclear Weapons Comparison. Source: Los Alamos National Laboratory (LANL)
Nuclear fusion is basically the opposite of fission — it is the process by which two light atomic nuclei combine to form a single, heavier one while releasing massive amounts of energy.
For fusion bombs, the nuclei of two extremely rare isotopes of hydrogen — deuterium and tritium — are fused together under extremely high temperatures and pressure, thus giving these bombs the moniker of hydrogen bombs or H-bombs.
While being way more destructive, fusion bombs pose some major challenges, the first of which is obtaining adequate fusionable material itself.
Moreover, the amount of energy that is required to create conditions of a fusion reaction is immense. Thus all H-bombs are basically two bombs in one — a fission bomb which produces adequate heat and pressure, to trigger the fusion reaction.
And what happens when a nuclear device is actually exploded?
Nuclear weapons are thousands, if not millions of times more powerful than conventional explosives like TNT.
Upon explosion, they produce four types of energy:
- A blast or a shock wave that can flatten and obliterate any physical structures in the blast radius;
- Bright light, which can cause permanent blindness even many kilometres away;
- Intense heat, which can literally turn human bodies into ashes in an instant; and
- Radiation, which includes both initial radiation, produced within a minute of detonation and residual (or delayed) nuclear radiation, which is emitted over a period of time.
More Explained
EXPRESS OPINION
May 10: Latest News
- 01
- 02
- 03
- 04
- 05