This post is the second of three articles first published by Xiaoduo Media in “Front Vision”. Front Vision is a Chinese online science magazine for children. My original English text produced with permission.
War and fascism – everything changes
The research described in the previous chapter involved scientists from across Europe, all sharing information and working towards a common goal. Through scientific papers, they shared their ideas equally, allowing people in many countries to contribute. But In 1933 Adolf Hitler took power in Germany. His fascist ideology, based on lies and hatred, blamed Jewish people and other countries for holding back Germany. Between 1933 and 1939 he and other fascist leaders started to discriminate against Jews and it was clear to all that even more terrible things were coming. Many Jewish scientists left Europe for America, to escape persecution. For example, Otto Frisch and Lise Meitner were refugees in Sweden fleeing the Nazi takeover of Austria when they identified nuclear fission.
Another Jewish refugee was Leo Szilard. In 1933 crossing the street in London he had a vision. If slow neutrons could induce nuclear reactions in elements, what if such a reaction created two neutrons? And each of those neutrons in turn made another reaction? 1-2-4-8-16-32-64-128 .. It grows and grows – a nuclear chain reaction. In 1939 (now safe in the USA) he heard of the discovery of fission in Uranium and realised the possibility of creating a fission chain reaction. Nuclear fission, theoretical calculations suggested, released unusually large amounts of energy. A nuclear chain reaction involving fission would suddenly release massive amounts of energy – it would be a bomb of enormous destructive power.
Szilard wasn’t the only person to realise the potential of nuclear fission chain reactions. In the UK – from September 1939 at war with Germany – James Chadwick (the discoverer of the Neutron and student of Rutherford), led serious investigations into the practicalities of making a nuclear bomb. At the same time, the German government started it’s own investigations, led by Werner Heisenberg.
In the USA in 1941, researchers created Plutonium from Uranium. They kept their discovery secret. Theoretical studies suggested that Plutonium, like Uranium, was fissile (could undergo nuclear fission) and could be produced in large quantities from Uranium.
How to make an atomic bomb
By 1941, the basic theory of how to make an atomic bomb was known to many physicists. What’s required is to generate a nuclear chain reaction involving nuclear fission. Two materials were known that did this, Plutonium and Uranium. However only one of the isotopes of Uranium was suitable, Uranium-235. Natural Uranium contained a mix of Uranium-235 and Uranium-238 and nobody knew an easy way to separate them out.
If you somehow managed to get hold of enough of either material, you needed to create a ‘critical mass’ of it. This is where a large enough volume material is tightly packed together such that not too many neutrons escape out, but instead enough hit other fissile atoms and create yet more neutrons and so sustain a chain reaction.
The concept of critical mass can be understood by thinking of a forest fire. If a tree standing alone in a field is hit by lightning and catches fire it releases heat, but this is lost to the atmosphere and soon the fire stops. This is like fission in a small mass of Uranium, a reaction starts but the neutrons don’t hit more fuel and so fission stops. If the first tree is closely surrounded by dead wood and other trees, the heat it produces heats this wood and causes them to start burning – it starts more chemical reactions – and so the fire spreads growing hotter and bigger. This is like a fission reaction starting within a critical mass of Uranium, most neutrons hit more fuel, creating ever more reactions. Nuclear fuel releases much more energy than wood, so things move much more quickly. This would release huge quantities of energy and so create an extraordinary explosion.
Physics is not engineering. Creating enough fissile material and turning it into a working bomb were huge challenges.
Manhattan project
In December 1941, Japan attacked a US naval base called Pearl Harbor and the USA entered the Second World War. Japan and Germany were aligned, so the USA declared war on both Germany and Japan.
Alerted by a letter from Leo Szilard and Einstein, the US government had already started research into nuclear weapons. Knowing of German expertise in nuclear physics, the goal was to create a bomb before Germany did. Now at war, a new US project was created and given the code name of the Manhattan Project. It had enormous resources available to it.
The project called on the best scientists in the world. The best physicists in American universities, like Philip Oppenheimer and the young Richard Feynman were called up in secret. Leo Szilard and Enrico Fermi and others had already fled fascism to reach the USA, and the British project team was merged in as the two countries became allies. In time Niels Bohr was involved, having fled his native Denmark in secret to escape Nazi persecution.
The project brought together different cultures. The many scientists who had fled to the USA from Hungary were called ‘Martians’. This was a joke name as – like aliens from another planet – they spoke an incomprehensible language and were incredibly intelligent. University scientists didn’t always get along with Army engineers. In his memoirs, General Groves – the leader of the Manhattan project – talks of a meeting where scientists gave him their estimate for how much Plutonium was required to make a bomb. Like an engineer, he assumed an estimate was one with maybe 25% or 50% uncertainty. However to physicists a reasonable estimate was one within a factor of ten.
Those on the project were completely focused on one goal, and solving the many technical challenges that faced them. Knowing that there were two types of bomb, based on Uranium-235 or Plutonium and not knowing which would be best, they decided to build both at the same time.
Little Boy, the Uranium weapon
The Manhattan project managed to secure large supplies of Uranium ore, but this contained a mix of Uranium-238 and the more fissile Uranium-235. These different isotopes are chemically identical, making separating them (a process called Uranium enrichment) a huge job. A huge secret site was built called Oak Ridge to focus on Uranium enrichment.
Various different approaches were considered. While chemically identical, U-238 is slightly heavier (because each atom contains more neutrons) so each method finds ways to use the weight difference to separate them.
The first method is to spin the Uranium in centrifuges. These spinning containers separate out the lighter U-235. Attempts by the Manhattan project to build machines failed, but this technique is now the preferred method.
The electromagnetic technique spun Uranium gas through a magnetic field to separate the isotopes. It required huge quantities of copper which was hard to find during wartime. In the end 13 tonnes of silver kept for use as money was used instead. This technique was inefficient, but used known technology and so was used in case the other techniques failed.
The Gaseous diffusion technique relies on the fact that lighter gas diffuses more quickly through a membrane. Huge buildings, four stories high and 0.8km long were built and the technique was very successful. The gas Uranium Hexaflouride was pumped under high pressure into a tube surrounded by a barrier, a membrane that contained thousands of tiny holes. Molecules made of the slightly lighter Uranium-235 (shown as orange dots) could more easily pass through these holes leaving behind gas contained more Uranium-238 (blue dots). This process separated the gas into a enriched stream and a depleted one. The enriched stream would still contain lots of Uranium-238, but it could be passed through into another set of pipes and through another barrier again and again, becoming each time more and more enriched. The depleted steam could be discarded – it was the Uranium-235 that was needed.
(See https://www.atomicheritage.org/history/isotope-separation-methods for some diagrams of this process)
Finally, the thermal diffusion technique placed hot Uranium gas in 15m tall tubes. The lighter Uranium-235 gathered more at the top – this was another effective way of separating out the isotopes by weight.
The three techniques were used in sequence, to create more and more concentrated Uranium-235. Eventually about 50 kilograms of uranium enriched to 89% uranium-235 was delivered to Los Alamos by July 1945 where it was turned into a bomb.
The design was simple, a little like a gun. A long tube contained two pieces of enriched Uranium. Each piece alone was too small to sustain nuclear fission reactions. Conventional explosives fired one piece down the tube into the other. This joined mass was big enough to become critical and create a nuclear fission chain reaction and so an explosion. The bomb had the codename “little boy”.
Fat man – Plutonium weapon
Despite being only discovered the year before, Plutonium was seen as another potential fuel for a bomb. Plutonium could be produced from un-enriched Uranium, by generating a controlled nuclear fission reaction. Nuclear fission reactions would produce neutrons, slowed by graphite blocks. The material that slows the neutrons is called the moderator, as it moderates (slows) the speed of the neutrons. Some of these neutrons sustain a steady rate of reactions, others turn Uranium into Plutonium. The rate of reaction was controlled by adding rods that absorbed neutrons to slow or even stop the reactions. The designs ensured there was no ever-increasing chain reaction as was wanted in a bomb, just a steady rate. The reactions produced heat, but the real purpose was creating Plutonium. Importantly, the Uranium fuel could be unprocessed ore – there was no need to enrich the Uranium-235 first.
Enrico Fermi, piled up Uranium oxide and graphite blocks in a squash court in a sports ground at Chicago University and so in 1942 created the first (man-made) self-sustaining nuclear reaction in Chicago Pile-1, the ancestor of all nuclear reactors. Soon a small secret town called Hanford was built to house a number of bigger nuclear reactors all dedicated to producing Plutonium. Once Plutonium had been produced, it could be chemically separated from the Uranium material.
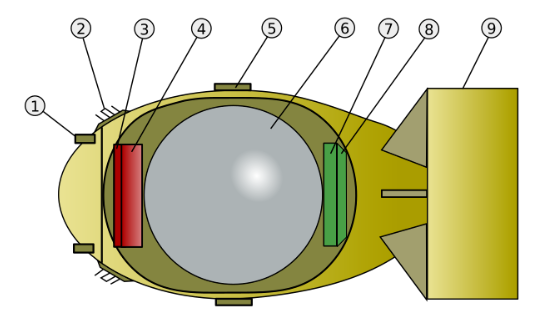

The design of the weapons was done at a site called Los Alamos. This required intense work from many of the world’s best physicists. To know if the bomb would work, they had to understand how it would work. To start with they calculated the energy released by a single fission reaction. This required knowledge of the exact mass of the material before and after, to calculate the missing mass converted to energy according to the Einstein equation.
That was the easy part. To create a sustained chain reaction, a large amount of fissile material needs to be suddenly pressed together to make a critical mass. A problem with the Plutonium was that it contained a mix of different isotopes, meaning the fission reactions acted in a different way. The material produced within the reactors contained more plutonium-240 (a very reactive isotope) than expected. This would start fission reactions much sooner than the plutonium-239. If a gun design was used, like in Little Boy, then the plutonium-240 would fission early and push the Plutonium apart before most of the material – the plutonium-239 – could fission. This would cause the bomb to fail to explode properly.
So a different and more complicated bomb design was required, one that involved taking a sphere of Plutonium and surrounding it with conventional explosives. The physicists had to write equations to model the behaviour of the Plutonium pressed together by explosives, then track the fission reactions as a critical mass was formed. Imagine trying to understand in detail what happens in the heart of a bomb, and then add on the complexity of understanding the fission reactions. Many equations and long difficult calculations were required and because Plutonium behaves different from Uranium, all the calculations were different from those required for the Uranium bomb design. The concept was simple but the design was complicated. The explosives were made with a complicated three-dimensional shapes to focus the shock wave through them and down onto the ball of Plutonium. The yellow detonator, started different conventional explosions with the outer layer of explosives. The curved shape at the based helped join the different shock waves into a single spherical wave that was then increased by two further layers of conventional explosives. This then compressed three different layers, of Aluminium, plastic and then Uranium, each with a different purpose.
Inside all this, the red Plutonium contained a polonium-beryllium initiator, a device that when compressed provided a good source of neutrons to initiate the chain reaction. The size and round shape of the bomb compared to ‘little boy’ led to it being called ‘fat man’.
They ran tests of the explosives (without any Plutonium) and used X-rays to see what happened inside, but still any big mistake in these calculations and the bomb wouldn’t work.
German efforts
Scientists who before the war had cooperated now found themselves on opposite sides of a vicious war. Non-Jewish German scientists like Werner Heisenberg might not be supporters of Hitler’s fascism, but most chose to stay and support their country as it fought a war.
Heisenberg was asked to lead efforts in Germany finding uses for nuclear fission. He understood as well as anyone the possibility of making a bomb, but focused mostly on the potential for energy generation, rather than weapons. Some people speculate that he did this as he didn’t want to give Hitler such a powerful weapon, but no-one knows for sure.
The Manhattan Project was started precisely to get a bomb before Germany, but nobody knew that Heisenberg would fail to deliver. To slow down any German efforts, the UK army, working with locals, attacked a site in Norway that was a source of heavy water, useful for nuclear research. In 1944 Heisenberg gave a talk in Switzerland, a neutral country. A US agent was sent with instructions to kill him if the talk suggested that Germany was close to having a bomb, but of course there was no need.
Testing and use in war
By 1945 the Manhattan project had achieved so many things. It had produced enough Uranium-235 for a single bomb and larger quantities of Plutonium. We’ve seen how complicated the calculations required for bomb design were – what was needed now was a test, to see if nuclear weapons actually worked.
A version of the fat man weapon was built and a site deep in the desert chosen. At 05:29 on the 16th July the device was exploded at the Trinity Test site. A huge flash of light lit up the desert, with colour changing from yellow, red to purple. A ball of fire slowly rose leaving behind a column of smoke, forming a characteristic mushroom shape. After about 40 seconds the blast wave reached the observers. Enrico Fermi dropped a series of pieces of paper and saw how far they were blown by the wind. He had already made a rough calculation and from the distance the paper was blown he estimated the size of the explosion to be the same as that created by exploding 10,000 tonnes of TNT.
The bomb contained 6.2kg of Plutonium of which 1kg was transformed by the fission reactions. Of that mass, about a gram was converted directly into energy. Enough energy to turn the desert sand beneath into glass.
The initial reaction of the scientists watching the test was joy at their success, but slowly they became solemn as they realised how terrifying a weapon they’d created. The blast wave spread outwards and was heard over a hundred kilometres away. A fake cover story was invented to explain it as the new bomb was still secret. The political implications spread around the world.
Nazi Germany had been defeated a few months earlier, but the war against Japan continued. The Japanese armed forces had been mostly defeated outside of Japan but refused to surrender. Faced with the prospect of having to invade Japan the US Army estimated that this would mean half a million US soldiers and 5 million Japanese people would be killed. The American president turned to his new atomic weapons as an alternative. On 6 August 1945 the Little Boy Uranium-235 weapon was dropped on the Japanese city of Hiroshima. Three days later a Plutonium fat man weapon was exploded over Nagasaki and six days after that, Japan surrendered and the war was over. Many of the scientists who worked on the Manhattan project argued the bombs should not have been used and were appalled that 10,000s of civilians had been killed by what they had built. The alternative argument is that they ended the war early and so saved more lives that way. Probably people will never agree which interpretation is correct.

Aftermath and impact on science and beyond
Nuclear physics moved in only a few years from experiments on tables in laboratories to a huge engineering project. Theoretical physicists would produce equations that within days were being used to design new machines and industrial processes.
Some of those who worked on the Manhattan project moved back into basic research. Having demonstrated it’s practical value, physicists had little difficulty finding the funding for building the bigger and bigger machines required to explore deeper inside the atom. The search began to find out what smaller particles neutrons and protons were built up from.
Some others stayed in weapons research. The process of fusing small atoms together – nuclear fusion – produces even more energy than fission and within 10 years a fusion weapon – the hydrogen bomb – had been made and tested. Others focused on more peaceful uses of nuclear fission. This included fields like medicine but many thought of the heat produced by the Manhattan reactors and saw it as a source of cheap energy.
1 comment
Comments are closed.